Oxygen Critical to Practical Islet Transplants
Teams from Universities of Arizona and Minnesota Collaborate


We spoke with Klearchos Papas, PhD about his oxygen generator-based islet ‘tea bag’ transplant device for which the JDRF just funded a pre-clinical study (release). Dr. Papas (bio) is a professor in the Departments of Surgery, Medical Imaging, and the BIO5 Institute at the University of Arizona College of Medicine – Tucson. He is also collaborating with the Center for Magnetic Resonance Research (CMRR) with Dr. Michael Garwood at the University of Minnesota.
“We call it a tea bag because it consists of two side-by-side layers of islets that are enriched with oxygen. It is a tiny device which when implanted under the skin senses glucose levels and releases insulin when needed.”
Why Oxygen is Important
For islets to survive the body’s immune system, the implanted islets need to be isolated from any direct contact with any cells from the recipient, including all white and red blood cells.
At the same time, islets need oxygen to function.
A person requires about 8300 islets per kg of body weight to produce sufficient insulin to manage their glucose. Thus, a 160 pound (72.5 kg) person would require about 600,000 islets.
Dr. Papas and his team showed that putting islets in an oxygen-rich environment enabled each islet to survive and thrive. The net result was that each islet produced more insulin, which reduces by 2-to-4 fold the number of islets needed to produce sufficient insulin for a person.

Oxygen also enabled the islets to be packed more densely in the same surface area thus reducing the total footprint of the implant. Today, Dr. Papas thinks the tea bag will eventually be about one-half the size of a credit card.
Interestingly, the UA team did not work with mice because the mouse islet dose is only 1/100th of a human dose. Instead, they worked with rats, which are larger and require a human islet dose that is 10% to 20% of an adult human dose.
Working with rats allowed for testing realistic islet densities in a small footprint device. This made it practical to move to swine next for their preclinical testing.
This large animal testing is the work that the JDRF is funding in which Klearchos Papas, Tom Loudovaris, and Gregory Korbutt are co-principal investigators.
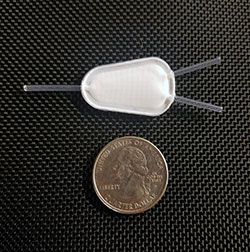
Tea Bag
The tea bag is made from Teflon, which is very durable and has a long history of safe use in humans. This Teflon material has pores. There are several layers of the Teflon membrane, each with different pore sizes.
The more porous outer membrane enables vascularization of the tea bag surface while the less porous inner membrane prevents direct cell-to-cell contact to protect the islets from the body’s autoimmune system. All the membrane layers allow pass-through of nutrients, insulin, glucagon, and other hormones.
Oxygen Supply
Islets in the pancreas are spheres containing about 1500 cells. Each cell within that sphere has access to microvessels penetrating the sphere delivering oxygen and glucose and extracting insulin. When you isolate islets for transplants this micro-vascularization is disrupted.
Without this intra-islet micro-vascularization, the vascularization of the surface of the implanted device is the sole means for oxygen supply, which is inadequate. Without supplemental oxygen, the surface area of the implanted islet device would need to be the size of a flat screen TV.
Installation, Durability, and Maintenance
The subcutaneous location of the tea bag makes installation and replacement a simple office procedure.
The life expectancy of the islets and the tea bag is expected to be between 1-3 years but theoretically, it could last a lot longer, even up to 10-15 years.
It is conceivable that a booster of islets could be installed in an already-implanted tea bag.
Oxygen Generator
During initial clinical trials, a wearable oxygen generator is anticipated.
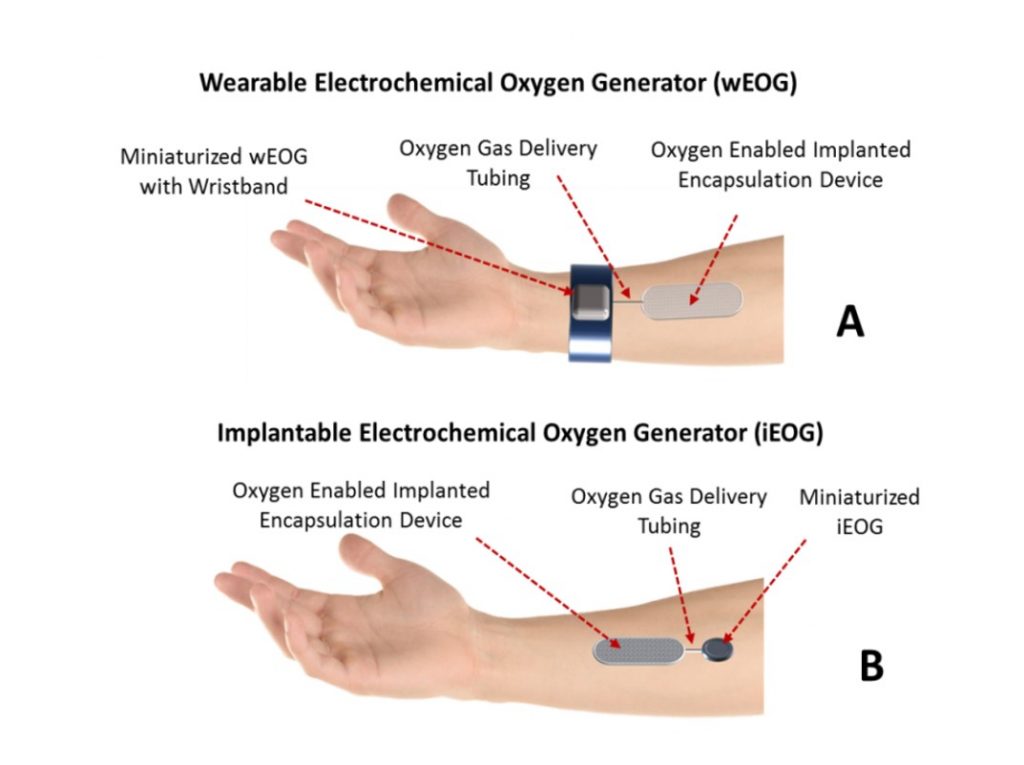
Development of an implantable generator does not require new science but it will be expensive to miniaturize the device and enable recharging of its batteries outside the skin via some field effect approach similar to modern phone chargers. This work is being done by a partner outside of the University of Arizona.
The target size for the implanted oxygen generator has a volume of 3 to 4 dimes, of which most is the battery. Recharging of the battery would be done every 1-2 days probably by wearing a wristwatch wirelessly charging the implanted oxygen generator while the patient is asleep.
Baxter Team

Baxter Healthcare developed a flat sheet immunoisolation device called TheraCyte and demonstrated that it protected human cells transplanted within it in human patients from rejection without immunosuppression. This was produced in the early 1990s but spun out the technology in 1999.
This device was ahead of its time and very promising but unfortunately, without the enhanced oxygen supply it would have to be very large (the size of a 50in flat screen TV) to provide enough insulin to reverse diabetes in a human. This is one of the major reasons why it has not been successfully utilized to treat patients with type 1 diabetes as of yet. However, combining a device like this with enhanced oxygen delivery will address this issue enabling a human islet dose to reverse diabetes in a device that is small and practical.
Dr. Papas has recently recruited to the University of Arizona team Dr. Robert Johnson (bio), Steven Neuenfeldt, and Dr. Tom Loudovaris who are key contributors to the development of the Baxter Theracyte device and are expected to contribute to the final product device development as it closely translates to clinical applications.
Children are Focus
The first clinical trials for the tea bag and oxygen generator will focus on the use of human islets from cadaveric donors to be transplanted in type 1 diabetes recipients without immunosuppression. If successful, this will pave the way to use this approach in children with type 1 diabetes.
Positioned for Partnerships
The University of Arizona and Dr. Papas have formed Procyon Technologies LLC in anticipation of forming a privately funded partnership to commercialize the products.
Early stages of the research have been supported over the past several years by other JDRF grants and NIH/ NIDDK (with the DP3 grant—DK106933).
Other project collaborators include Bernhard Hering, MD, of the University of Minnesota; Sean Limesand, PhD, of the UA College of Agriculture and Life Sciences and UA College of Medicine – Tucson researchers Leigh Neumayer, MD, Leah Steyn, PhD, Ron Lynch, PhD, and Robert Harland, MD.