Medtronic Issues Wide-Ranging Infusion Set Recall
9/11/2017 – 12:30 pm (EST) – This story has been updated.
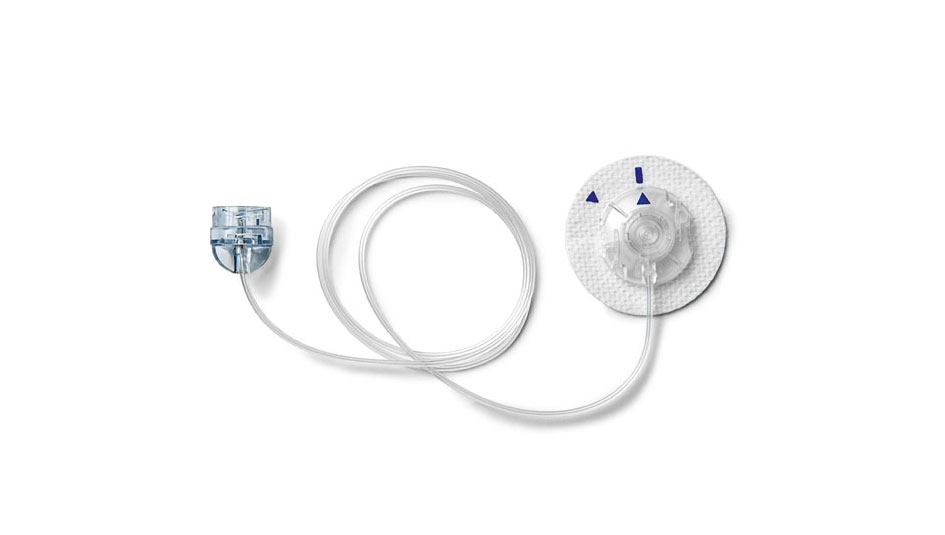
On September 11th, Medtronic issued a notice stating that the company is voluntarily recalling certain lots of infusion sets used with all models of its insulin pumps. The recall covers all lots of Medtronic infusion sets sent to customers before April 2017, except for its infusion sets with luer locks during that time period; this exception was first reported by Diabetes Mine and has been confirmed by Medtronic. The recall also may affect some lots of infusion sets shipped after April 2017. A Medtronic press release states that the recall involves a discontinued component that has since been replaced in infusion sets currently being sold by Medtronic.
Medtronic undertook the recall after field reports suggested that a vent membrane in the infusion set could become blocked by fluid during the priming and filling process. This can increase the risk for overdelivery of insulin during pump use, which could in turn increase the risk of hypoglycemia in the pump user, according to the press release. Currently, Medtronic has only received reports of this happening in one out of every 2 million infusion sets in use, but that ratio very well might go up somewhat as news of the recall spreads.
Medtronic reports that it has developed a membrane design update which the company believes will reduce the risk of overdelivery of insulin after a set change. This update was phased into infusion set lots shipped after April 2017.
The company states it will replace infusion sets covered by the recall free of charge, and it discourages customers from using infusion sets from lots listed in the recall. In the FAQ section about the recall, the company states users should consider using multiple daily injections while waiting for infusion set replacements rather than use the affected infusion sets. It also, not coincidentally, provided a link to its guide for how to best change infusion sets to minimize the risk of insulin overdelivery.
Rather than listing the lots covered, Medtronic has instead provided a url for determining whether an infusion set lot is covered by the recall. Those who wish to check their infusion sets should go to https://checklots.medtronicdiabetes.com/home. There they will be instructed to select their home country, verify their age, and input their infusion set information. Pamela Reese, a spokesperson for Medtronic, encouraged all Medtronic infusion set customers to check their lots on the website, even if they think their infusion sets may not be covered.
Those who wish to learn more offline are instructed by Medtronic to call 1-888-204-7616, a number specifically dedicated to the infusion set recall. However, as of 10:30 am (EST) on September 11th, a recording first encourages callers to go to the url; only after the recording finishes this initial message and there is a pause is a user given the option to stay on the line to speak to a Medtronic representative.
As Insulin Nation learns more details about this recall, we will provide updates.
Do you have an idea you would like to write about for Insulin Nation? Send your pitch to submissions@insulinnation.com.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.