Tandem Closed Loop Cleared for Pediatric Patients
Large-scale pediatric study secures FDA approval for use of Tandem Control-IQ hybrid closed-loop pump in children as young as 6
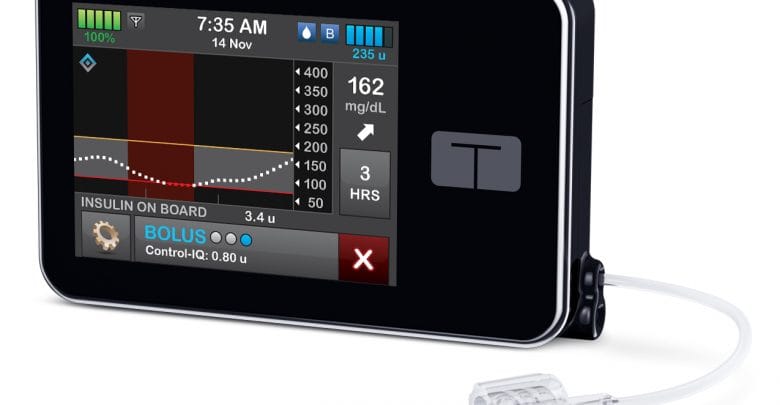
Tandem’s Control-IQ, an insulin pump that works with Dexcom’s G6 CGM to automatically control blood sugar, has been widely celebrated among users since it first became available in early 2020.
This pump was originally approved for use by people with type 1 diabetes over the age of 13. It has now been shown to be safe and effective when used for type 1 children as young as six.
This new FDA approval comes with the release of a multicenter, randomized, 16-week pediatric trial.
Achieving Healthy Blood Sugars in Children is Difficult
Type 1 diabetes is a difficult thing to manage no matter how old you are, but it is especially hard for young children and the parents responsible for their care.
Fewer than 20% of children are able to successfully keep their blood sugars within a healthy range.
This creates problems both short term and long term.
- Fluctuating blood sugars can quickly lead to deadly hypoglycemic events, especially overnight.
- CGMs can reduce this risk by alerting caregivers to problems before they become serious, but do nothing to relieve the burden of care inflicted on parents.
- Children are especially prone to hyperglycemia, which can be caused and complicated by common childhood illnesses.
- High blood sugars that are not brought down quickly, or that occur during illness can easily progress to diabetic ketoacidosis.
In addition to the acute effects of poorly-controlled diabetes, consistently high and frequently fluctuating blood sugars can lead to long term complications such as neuropathy, heart disease, and kidney problems.
All these complications are more common and tend to begin earlier in life in patients who were diagnosed in childhood.
Tandem Loop Lowers Treatment Burden for Parents
Finding a way to better control blood sugars in T1 children while simultaneously reducing the management and treatment burden of the disease has been a dream of doctors and type 1 parents for a long time.
With the release of the newest trial data from Tandem, it seems we are finally getting closer to achieving such a goal.
The study, which was published in The New England Journal of Medicine, enrolled 101 children from multiple diabetes treatment centers across the US. The children, all between the ages of 6 and 13, were randomly assigned to the control group or the experimental group.
Those in the control group were given either 1) a standard pump and separate CGM or 2) a pump enabled with Tandem’s Basal-IQ (which automatically lowers insulin rates to avoid hypoglycemia but does not otherwise automatically adjust basal rates).
Those in the experimental group received the newer Tandem Control-IQ enabled pump, which uses information from the Dexcom G6 CGM to automatically:
- Adjust insulin basal rates to keep blood sugars between 70 mg/dL and 180 mg/dL
- Increase insulin rates when a high blood sugar is predicted
- Decrease insulin rates when a low is predicted
- And give a correction dose for missed meal boluses
All subjects were given a run-in period to get used to their specific new pump set up before being monitored for 16 weeks.
Control-IQ Means More Time In-Range for Type 1 Children
Researchers found that the experimental group using the Control-IQ had significantly better blood sugar control than the group utilizing traditional pumps and CGMs.
Overall, the experimental group saw time in range increase 7% during the day and a 26% overnight, compared to the control group.
In both groups, time in hypoglycemia (below 70 mg/dL) was low:
- 1.6% median time in the Control-IQ group
- And 1.8% in the traditional pump group
Time in hyperglycemia was significantly lower in the experimental group, however:
- The Control-IQ group sat above 180 mg/dL an average of 31% of the time
- While the traditional pump group was in hyperglycemic territory 43% of the time
The mean glucose over the course of the 4-month study was also significantly lower in the experimental group:
- 162 mg/dL for those using Control-IQ
- Versus 179 mg/dL for those using traditional pumps
The experimental group saw an increase in average time in range from 53% at baseline to 67% by the end of the study period. Meanwhile, the control group only saw a 4% increase in time in range between the beginning and end of the study.
For the Control-IQ group, this equates to an increase of 3.4 hours per day spent in range.
But the most significant difference in time in range between the two groups occurred at night with the experimental group showing an average of 80% time in range while the control group showed just 54%.
Most importantly, the positive results of using Tandem’s Control-IQ appeared to be consistent across different ages, BMIs, genders, household incomes, parental education, and baseline A1c.
- The benefits of using the automated pump could be seen in subjects familiar with traditional pump therapy as well as those who had never used a pump before.
- Additionally, the increase in time in range was seen immediately after the first month of treatment and continued for the full study period.
These stats, specifically, speak to the ability of Tandem’s Control-IQ to reduce management burden while simultaneously improving the long term prognosis for children living with type 1 diabetes.