In-depth on Tandem’s Advanced Hybrid Closed-Loop System
Tandem’s FDA-approved Control IQ system is a major improvement over other loop systems and is now on the market in the US
In a win for all people with type 1 diabetes (even those who won’t directly benefit from this closed-loop system), the FDA has approved Tandem’s new Control IQ software for release.
This new automated glycemic control algorithm is just the second hybrid closed-loop system available to patients with an FDA clearance. And it promises tighter control, easier usability, and more customization than the current 670g automated system from Medtronic.
How Control IQ Works
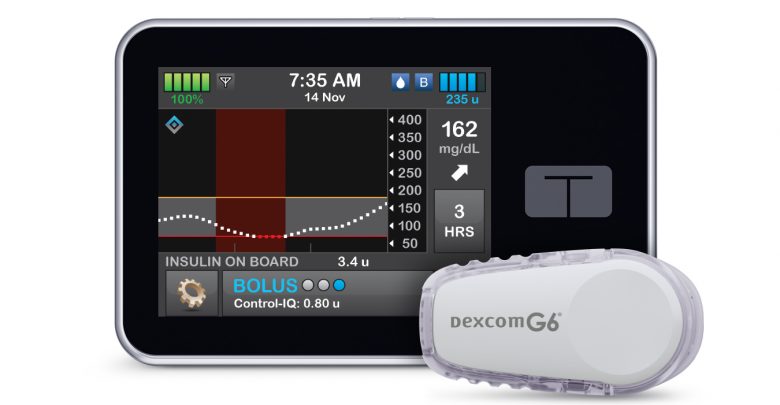
Like other automated pump systems, Tandem’s new algorithm works by pairing an insulin pump with a continuous glucose monitor (CGM) to allow for automatic insulin adjustments based on predicted glucose values. In this case, Tandem’s t:slim X2 pump and Dexcom’s G6 CGM will work together to keep blood sugars within the ADA recommended range of 70 to 180.
This is accomplished by multiple pathways.
To help avoid hyperglycemia, the automated system will increase basal insulin when blood sugar is predicted to rise above 160 within 30 minutes. If blood sugar rises above 180 the system can deliver a correction dose to quickly bring sugars back in range.
On the other end, Control IQ will automatically reduce basal insulin if blood sugar is predicted to drop below 112.5. If sugars are predicted to drop even lower, below 70, the system will suspend insulin delivery altogether until sugars begin to rise again.
The system also allows the user to set temporary glucose goals for times of increased activity to help avoid lows.
In a study published in the NE Journal of Medicine, this combination of control factors led to an 11% increase in time spent in range and a 2.6-hour daily decrease in high blood sugar, on average. These types of positive results were seen across a wide range of ages and baseline A1Cs.
What Sets This New System Apart
Control IQ is a huge improvement over Tandem’s current automated system, Basal IQ. This current option automatically suspends basal insulin in the event of a predicted low but does not include any of the new measures to reduce hyperglycemia.
While there are a few systems that integrate this type of semi-automated glucose control, Medtronic’s 670g system is currently the only other hybrid closed-loop system approved for use by the FDA.
While the 670g and new Control IQ share many of the same features, there are some key differences that clearly give Tandem the edge.
First, and likely most important to those using the system, Control IQ works with the Dexcom G6 CGM and requires no finger pricks to function in auto mode. Compared to the 670g, which requires a minimum of two blood meter calibrations every day, this is a major advantage.
Beyond a reduced need for finger pokes, Control IQ also has the capability of auto-dosing correction boluses for high blood sugar. If sugars rise above 180 and active insulin levels allow, the system will automatically give 60% of a normal correction dose with the goal of bringing glucose levels down to 110. This is something that Medtronic’s hybrid closed-loop system cannot do.
Overall, the Tandem system is much more aggressive than Medtronic’s.
While the 670g shoots for a blood sugar of 120 throughout the day and night, Control IQ works toward a goal of 110 during the day and works more aggressively overnight to keep your sugars tightly between 112.5 and 120.
Despite the more aggressive algorithm, neither auto-mode enabled system is right for everyone.
While both systems set goal blood sugars that would equate to an A1C in the 5’s, the actual blood sugar averages tend to be much higher. Medtronic’s original study reported an average A1C of 6.9 while Tendem’s trial showed an average A1C of 7.1 after six months.
For people living with diabetes who currently have A1Cs lower than 6.5, it is unlikely either system will provide much of a benefit in terms of control. But for those struggling with their numbers either option is likely to improve your management. And given the extra capabilities and apparent ease of use, Tandem’s Control IQ has the potential to do even more than the current hybrid loop option.
Looking Beyond the Numbers
Despite all the potential packed into the new Tandem system, the true excitement behind this launch may have less to do with the system itself and more to do with how Tandem plans to utilize it.
Control IQ is the first automated glucose control device that has been designated as “interoperable,” meaning it can be used for multiple devices without undergoing the full FDA approval process. While the upcoming launch of Control IQ includes only use with the Dexcom G6, Tandem is currently working with Abbott to make Control IQ pumps that will work with their FreeStyle Libre CGM.
By making this algorithm available across multiple device types through various companies, Tandem assures more people with diabetes can take advantage of their technology. According to Aaron Kowalski, President and CEO of JDRF, this type of open protocol system is important because “people with different devices could use the algorithm and manage their glucose levels in a way that works best for them.”
For those who already have a t:slim X2 pump, Tandem is offering a free software download to upgrade your system to Control IQ once it is available.
They also plan to make all future software upgrades immediately available the same way your cell phone can be updated without purchasing a new device. This is a big change from traditional software updates that require patients to purchase the newest model, something that most insurances will only allow once every four years.
It is impossible to say if this new hybrid closed-loop device will live up to our expectations.
It is fair to say that the release of Control IQ and the measures Tandem is taking to make the system more widely available are steps in the right direction for all of us in the diabetic community.
Note: On January 15, 2020, Tandem Diabetes Care Announced Commercial Launch of the t:slim X2 Insulin Pump with Control-IQ Technology in the United States.