Putting Killer Cells in Reverse: The Hidden Power of CXCL12
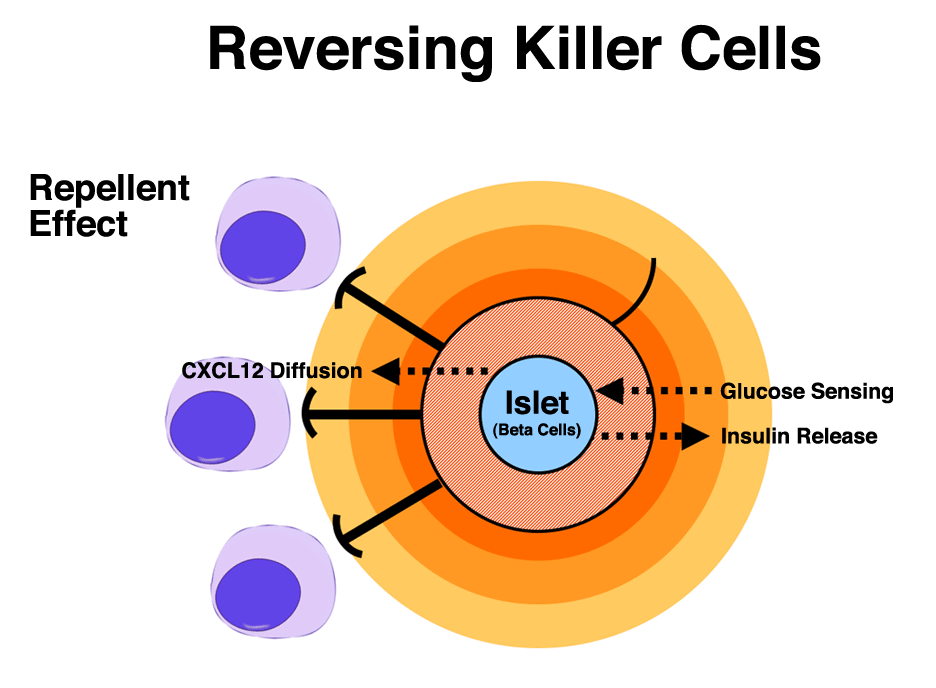
Think, for a moment, of our immune systems as a vehicle with forward, neutral, and reverse gears. Inflammation in the body puts the system in “drive,” mobilizing cells called lymphocytes, the infantry of the immune system. These lymphocytes go to the site of invasion and repel foreign marauders by killing them, thus fighting off infections and targeting cancers and other diseases. Discovering how to put the same “killer” cells in reverse, or directing them to avoid newly introduced cells without reacting, is a critical step in allowing transplants of healthy islets, containing insulin-secreting beta cells, into people with Type 1 diabetes. But the immune system has a complex transmission, and finding the best way to select “reverse” has encompassed much of the work of Dr. Mark Poznansky, an infectious disease specialist at Massachusetts General Hospital (MGH) for more than 13 years.
Poznansky has developed a process to keep “killer” cells away from transplanted tissue, specifically islets, by deceiving them into not recognizing new tissues containing beta cells as foreign. Islets, and the beta cells within them, are the part of the pancreatic system that regulate insulin and glucose levels, and are destroyed in patients with Type 1 diabetes. His theory, already validated in mouse models (publication of the results is pending), is headed toward large animal trials in Rhesus monkeys. Assuming those trials are successful, and the approach optimized, human trials will soon follow. The impact of his discovery on the survival and long-term health of transplanted islets into Type 1s – and the resulting remission of the disease – could be profound. It may prevent transplant recipients from having to take anti-rejection drugs that affect the entire body, also known as systemic suppression.

A Young Scientist’s Discovery

As a postdoctoral fellow working at Massachusetts General Hospital in 2000, Poznansky and his fellow researchers published a paper titled “Active Movement of T Cells Away From a Chemokine,” in Nature Medicine. The essence of the article was their discovery of a chemical signal that helped both to attract and repel immune cells. While the attraction of immune cells was fairly well understood at the time, the repulsion effect was not. According to an article written by Eugene Russo in The Scientist magazine, “Poznansky was convinced that the apparent ‘chemorepulsion,’ a phenomenon not nearly as well understood as ‘chemoattraction,’ was consistent and real.” More than a decade of further exploration has proven his hypothesis.
What Poznansky and the team members had discovered was that sufficiently high concentrations of CXCL12, a vital substance secreted by certain cells of the immune system, repelled killer T cells both in test tubes and in mice. The reverse signal was also conserved between mouse cells and human cells. Discovering the ability to actually repel these killer cells marks a significant step forward in the fight against diabetes, one that could potentially lead to substantially improved quality of life for those with the diseases or even life-altering cures.
Today, Dr. Poznansky, with a dual MD and PhD, is the Director of the Vaccine and Immunotherapy Center (VIC) housed at Massachusetts General Hospital and affiliated with Harvard Medical School, where he also serves as an Associate Professor. VIC focuses on translational medical research, shorthand for research that can move quickly from the scientist’s bench to the patient’s bedside. Or, in the case of Type 1 diabetes, into a product that can protect transplanted islets from the same sort of immune system attack that caused their diabetes in the first place.
The foundation of Poznansky’s discovery combines pig islets with small amounts of CXCL12 (immune-isolating microcapsules) in an alginate (seaweed derived) coating. Placed in the abdominal area of a Type 1 patient, the protected islets could do what they have already done in mouse models: cure diabetes. He looks forward to trials in Rhesus monkeys as the next step towards that goal.
Keep It Simple
Dr. Poznansky had no connection to diabetes when he began his work. “I’m an infectious diseases physician and immunologist and I was focusing on HIV and cancer at that time,” he recalls. “I just thought, in transplantation, that the issue is that all the immune cells go in and destroy something. Well, what if you could repel them? At that time (in 2000), islet transplantation was coming on the scene, and it seemed to me that it was approachable because you had these small clusters of cells that you could exert a repellant effect around and protect them from an attack by killer cells at the site of transplantion.” The effect of Poznansky’s discovery, and resulting therapy, is remarkably similar to the body’s reaction to unpleasant smells: your body instinctively and immediately pulls back. In mice, the killer cells have a similar reaction when they receive a repellent signal from CXCL12 – they go into reverse and pull away.
As he began to work with people affected by Type 1 diabetes, Poznansky became deeply sympathetic to their situation. “I go home on weekends and take breaks from doing research,” he says. “Many of these people are doing research on themselves every day of their lives. Others struggle with their condition, getting through each day by injecting themselves with insulin. I began to personally think that yes, the insulin injections and glucose testing are lifesaving, but there’s got to be something beyond that is much better.”
In his research, Poznansky stresses the value of simplicity. “I’ve learned in medicine that the simplicity of the product counts,” he says. “The complexity of islet transplantation was, firstly, that you injected them into the liver, and secondly, that you had to take lifelong immune suppression. Our new technology has the potential to be much simpler: you take islets, either from a donor human or pig pancreas, you coat them in a few hours’ procedure, and then place them under the skin, or in the abdominal cavity. It’s a day procedure, no overnight stay. It’s got to be that simple.”
From Bench to Bedside: The Investment Valley of Death
In the early days of genetic engineering all a venture capital firm needed to prompt an investment of millions of dollars was a set of clinical trials showing proof of concept, and sometimes not even that much development. The path to a commercial product is much more difficult today. Life sciences investors have participated in many failed “blockbuster” attempts, and now hold back until much later in the process. Not-for-profit funding from sources like the National Institute of Health and the JDRF (both of which have helped fund Poznansky’s research to where it is today) addresses some of the need, but there is an increasing funding gap that requires new layers of philanthropic donations to continue advancement. Often the federal government has been a solution, but current fiscal realities put continued support from that source in doubt. Additionally, there are very few researchers who combine the scientific talent and business savvy needed to bring a product all the way to market from the lab.

Recognizing these facts, VIC doesn’t expect its doctors and scientists to master the complex processes of capital funding and product development. Instead, it matches them and their projects to business professionals on VIC’s payroll, executives and professionals with MBA degrees and experience in life sciences investing. Dr. Poznansky’s business collaborator is Timothy Brauns, the associate director of VIC.
“Mark runs a traditional academic laboratory,” says Brauns. “They do animal work or in-vitro work in test tubes on interesting scientific problems. However, he also realizes that new drugs, targets and diagnostics need to be advanced in a different way than academic research might be.” Part of Brauns’ charge is to define the development path for new products and seek out funding opportunities and approaches that are outside of the typical not-for-profit sphere to support the progress of those products from bench to bedside. To advance medicine, his role is critical in today’s environment.
Poznansky is wholeheartedly dedicated to bringing a product to market sooner rather than later. “What dovetails with Tim,” he asserts, “is that I realized in the last few years that you have to make a determined attempt within academia to move a discovery towards what could be a product or else the full potential of the research may never be realized. There are 101 experiments we could do, but probably only a handful or even possibly one that you need to do to get to first base in humans. It’s very difficult in science, as an academic, to face “go, no go” decisions. We work in these fields for decades, believing that one day this is going to help somebody, but in the end very few of these things are effective therapies for humans.”
The Best and the Brightest
An intriguing aspect of Poznansky’s work is his lab’s inclusion in a newly created JDRF consortium of 17 research groups focused on islet transplants This consortium also includes the Diabetes Research Institute, about whose work Insulin Nation has written extensively (see various articles in our Cure Insight category). The fundamental purpose of the JDRF consortium, at this point, is not to create a project in which the best minds are harnessed under strict direction towards a goal with a specific timeline, like the Manhattan Project that developed the first atomic bomb. Rather, it is a first and valuable step towards avoiding needless repetition of experiments and duplicate model developments. Depending on what the participants learn from each other by sharing, a clear path might emerge that would chart a direct course to a successful protocol. In one possible scenario, for instance, Poznansky’sCXCL12 technology might be linked to DRI’s “BioHub” scaffold that can encompass a large number of islets for insertion into the body. This is purely a theoretical example, but it shows why the consortium approach might shorten the time needed to develop and test cure models in human patients.
“Mark formed VIC to take promising innovations like CXCL12 immune-isolating microcapsules and put them on a fast track toward development, to the point they could be successfully licensed,” Brauns points out. Taking more responsibility in the academic research community towards introducing marketable cure-focused products and procedures is today’s trend. It’s a welcome development for long-suffering diabetes patients.