Games Cells Play: Protecting Islet Transplants
Two childhood favorites are Hide and Seek, in which kids try to keep someone from finding them, and Trick or Treat, the Halloween tradition that involves presenting yourself as someone or something you aren’t. In the same vein, keeping transplanted islets alive includes finding ways to hide them, or to fool the body into accepting them as “self” so they will not be destroyed by the immune system. Scientists, such as Alice Tomei, Ph.D., at Miami’s Diabetes Research Institute (DRI), are developing techniques that may do both. Their goal: protect the beta cells inside transplanted islets from being rejected, since they’re foreign tissue, and from being destroyed by the immune system attack that caused the patient’s Type 1 diabetes in the first place.
“We know that Type 1 diabetes is an autoimmune disease,” said Tomei, one of the principal investigators in cell protection at DRI. “The body’s immune system mistakenly identifies the insulin-producing beta cells in the pancreas as foreign, and it attacks and kills them.” The same thing will happen if transplanted islets are not protected, because the new cells are foreign.
The Transplant is the Easy Part
Research trials at the DRI have shown that transplanting several hundred thousand donor islets — each a cluster of thousands of beta cells and other critical cells harvested from human cadavers — can reverse diabetes in both animals and humans. But the transplant itself is just the beginning of the challenge. In addition to immediately needing oxygen and nutrition post-transplant, the new islets need to be protected from the immune system.
Transplanting organs and tissues is a well-established technique. The traditional way to suppress immune system attack is to put the recipient on various combinations of anti-rejection drugs for the rest of his or her life. This systemic approach, in which the drugs are released throughout the body, may result in serious side effects, including risk of infections, cancer, increased cholesterol, hypertension, and ironically, in the case of diabetes patients, high blood sugar. And, the drugs are inappropriate for children except in extreme cases, meaning this therapy isn’t currently available to more than 25 percent of the Type 1 diabetes population.
“The current encapsulation technology involves beads that are a consistent size,” Tomei said. “Islets, however, are different sizes. In order to accommodate the largest islets, you end up with beads that are much too large for the smaller islets. Our conformal coating technology overcomes that problem. It involves running the islets through a polymer bath. This gives each islet a coating that is tight, with very little additional space, and which is the shape of the islet itself. It’s a lot like shrink wrapping.”
To avoid or minimize these problems, one approach has been to encase or encapsulate the islets within a protective barrier, hiding them from the immune system. This technique is straightforward enough in theory, but islets are also very irregular in shape and size, so the “standard” one-size-fits-all capsules used in testing, manufactured in a spherical shape, can be much larger than the islets. Empty space inside the capsule means greater distance and time for oxygen and nutrients to reach the islets. As a result, up to 60 percent of transplanted islets can die — either from immune attack, suffocation or general stress — before they can ever become established and begin producing insulin. The cells that remain also suffer, due to being overworked.
Shrink-wrapped Islets
Working with other DRI researchers, Tomei is taking on these challenges in two novel ways that provide localized, rather than systemic, solutions. The first is a cell encapsulation technique known as conformal coating, which is an improvement on the “standard” sphere-shaped encapsulation methods. This is the Hide and Seek strategy, through which the coating is used to mask the presence of the transplanted islets.
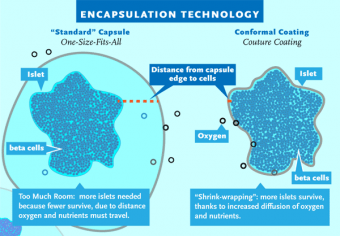 “The current encapsulation technology involves beads that are a consistent size,” Tomei said. “Islets, however, are different sizes. In order to accommodate the largest islets, you end up with beads that are much too large for the smaller islets. Our conformal coating technology overcomes that problem. It involves running the islets through a polymer bath. This gives each islet a coating that is tight, with very little additional space, and which is the shape of the islet itself. It’s a lot like shrink wrapping. Because this technique reduces the total volume of the islets prepared for transplantation, it gives us more options in terms of where in the body we can transplant them,” Tomei explained.
“The current encapsulation technology involves beads that are a consistent size,” Tomei said. “Islets, however, are different sizes. In order to accommodate the largest islets, you end up with beads that are much too large for the smaller islets. Our conformal coating technology overcomes that problem. It involves running the islets through a polymer bath. This gives each islet a coating that is tight, with very little additional space, and which is the shape of the islet itself. It’s a lot like shrink wrapping. Because this technique reduces the total volume of the islets prepared for transplantation, it gives us more options in terms of where in the body we can transplant them,” Tomei explained.

has diabetes, and she wonders if her
one-year old daughter may some day develop
it too, due to family history.
“The material we use is a hydrogel — it’s like a porous contact lens,” she continued. “It allows oxygen and food to get in and waste to get out; it’s a nice little casing for the islets. We have tested human islets and islets from other species and we have observed that these very thin coatings do not affect islet function, which is their ability to respond to glucose challenges by secreting insulin. We also have not observed any delay in insulin secretion when the islets are exposed to glucose. This delay has been one of our greatest challenges, and we have completely eliminated it. And now we have proof of principle in vivo; we have been able to reverse hypoglycemia when we have transplanted conformal-coated islets into rodents.”
Cleverly Disguised Cells
To further protect islets from the immune system, Tomei and her team are working on immunomodulation strategies, which come from her background in cancer research. Cancer cells surround themselves with a specific protein, known as CCL21, to trick the immune system into not killing the tumor. This protein disguises the cancer cells and makes them appear unthreatening. In Tomei’s work, the Trick or Treat strategy is being explored as a method of fooling the transplant recipient’s immune system into not recognizing the islet graft as foreign.
“We want to try to get the body to accept the graft, not reject it,” Tomei said. “In terms of immunomodulation, the skill is in how you apply the protein. In cancer, the cells express the protein from inside themselves. So the question is can we produce islets that express the protein, or do we somehow put the islets together with the protein when we do the transplant and develop a means to control the release of this protein?”
Both protective approaches are being studied on parallel paths. Tomei believes the answer ultimately may lie in using these methods together. “Each patient is different, and it may be desirable to have multiple options,” she said. “It’s the same approach we take to high blood pressure treatment. Some patients are fine with just one medication; others need to take a couple of them. The all have the same problem, but they are treated in different ways.”
Tomei, like so many researchers at the DRI, has a personal connection to diabetes; her aunt has the disease. And because Tomei also has a one-year-old daughter, she wonders if the family history might make her daughter more likely to develop diabetes. “Recently, I saw a little girl who had been brought into our clinic for treatment and she looked just like my daughter,” Tomei recalled. “Seeing that little girl inspired me to work even harder to find a cure.”