Nasal Glucagon Proves Effective in Latest Trial
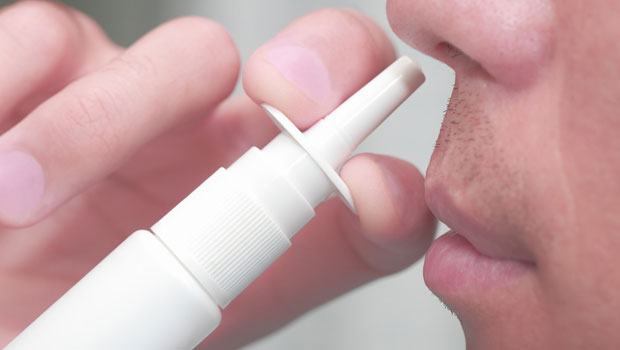
Anyone who has had to inject glucagon during a crisis would gladly reach for something easier to use, if it were available, but only if that alternative were just as effective in staving off hypoglycemia. Locemia Solutions has been developing an intranasal glucagon that offers the possibility of eliminating glucagon injections, and the company has announced a string of positive results in key safety trials for its product in 2015. The results of the most recent study, released in December 2015, showed that its intranasal glucagon could be used effectively by medical personnel to stave off hypoglycemia in adults with Type 1.
The study, conducted by a team of researchers led by Dr. Michael Rickels of the University of Pennsylvania, measured glucagon effectiveness in a laboratory setting. Researchers induced hypoglycemic blood sugar levels of less than 60 mg/dL in two separate occasions in 75 adults with Type 1 diabetes, and then had medical staff administer either experimental intranasal glucagon or the standard injectable glucagon, according to a report in MedPage Today. Success was based on whether the participant’s blood sugar level had risen by 20 mg/dL to 70 mg/dL within 30 minutes once the glucagon was administered.
Obviously, it would have been ideal if the nasal glucagon performed as well or better than the injectable glucagon, but the new glucagon just missed that goal by one trial participant and 10 minutes. During the study, all 75 participants that had injectable glucagon met that blood sugar rise goal within 30 minutes. Meanwhile, 74 out of 75 participants met that goal with the intranasal glucagon, and the one outlier met that goal within 40 minutes instead of 30 minutes. On average, there was just a three-minute difference between the two glucagons when it came to meeting that blood sugar rise goal.
The lead researcher, who is not affiliated with Locemia, attributed the one instance of failure to meet target to human error on the part of the person administering the nasal glucagon. One drawback to the study was that the new glucagon was administered by medical staff, which makes it harder to evaluate how easy it might be to use by those who didn’t have medical training. The MedPage report did cite a European study which showed that people with Type 1 and their caregivers (who lacked medical training) were able to use it effectively.
It’s impossible to know how FDA regulators will react to an application for approval for the new glucagon. However, Locemia Solutions might be able to argue that even if its glucagon were slightly less effective than injectable glucagon, it would still lead to better medical outcomes. That’s because many people elect not to inject glucagon in unresponsive individuals with Type 1, thus delaying medical treatment.
Lilly, the lead producer of injectable glucagon, has acquired the rights to market nasal glucagon. Lilly officials have not announced a date to go before the FDA for approval, however.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.