FreeStyle Libre Use Associated with Better Glucose Control
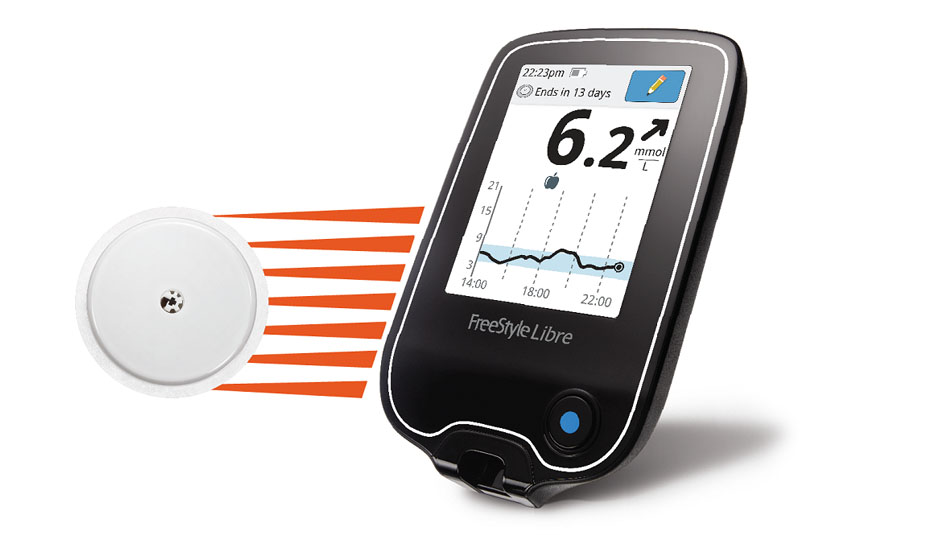
Knowledge may indeed be power when it comes to glucose monitoring, if a new analysis of more than 50,000 users of Abbott’s FreeStyle Libre is any indication.
As Abbott seeks approval for its glucose monitoring system, it has released data from Libre users to show how the device is being used and how it might contribute to improved glucose control in a real world setting. The analysis of user data aligns with previous clinical trial data on the Libre’s use and effectiveness.
Read “Mainly Prickless CGM Approved for Kids in Europe.”
Researchers found that Libre users checked their glucose readings an average of 16 times a day; that’s about three times the amount of fingerstick checks that medical professionals recommend for people with Type 1 diabetes, according to Tim Dunn, an Abbott associate research fellow connected with the analysis. The Libre differs from a continuous glucose monitor in that it doesn’t transmit readings at all times it’s activated. Instead, the system requires users to scan a sensor to get the most current glucose reading, a trend arrow, and an eight-hour history of readings. This extra step means it requires a more active buy-in from users, and Libre designers were gratified to see how often users opted in to get that data.
Read “FDA Approves Dexcom G5 as Replacement for Most Fingersticks.”
“I would have guessed eight or nine times; sixteen wouldn’t really have crossed my mind,” said Dunn. He later added, “It surprised me personally, and I was probably on the optimistic side.”
To come to this figure, Abbott researchers crunched 18 months of data from more than 50,000 Libre users. As part of the user agreement, Libre users must agree to have depersonalized information about their use of the device and their glucose levels transmitted to Abbott for analyses like these. In total, the researchers analyzed the trends of approximately 409 million glucose measurements taken during some 86 million hours.
The analysis also found that increased use of the Libre was associated with better glucose control. Those who scanned their readings the least had an average A1C score of 8.0, and those who scanned the most had an average A1C score of 6.7. Those who used the Libre the most also saw decreased amounts of time spent in hypoglycemia and hyperglycemia.
Dunn acknowledged that those who chose to use the Libre system represented a self-selected group, one that most likely is more motivated than non glucose-monitor users to be proactive about glucose control. He also was cautious to make clear that having access to glucose readings is just one step in glucose control. Still, he said that the trend lines in the data seemed clearly to indicate that a higher rate of Libre use contributed to glucose maintenance.
“Part of whatever they are doing is related to checking their glucose,” Dunn said.
This analysis closely mirrors clinical trial results for the Libre system. In one real-world study, the IMPACT study, participants either used a Libre to monitor glucose readings or were asked to use traditional fingerstick monitoring. Those using the Libre checked their glucose readings an average of 15 times a day. Libre users also saw increased glucose control over those who monitored levels by fingerstick. The study’s results align with continuous glucose monitor studies which also have shown improved glucose control over self-monitoring by fingerstick. Dexcom, for example, recently published study data showing that participants on multiple daily injections using a Dexcom CGM saw improved A1C control and spent less time in hypoglycemia and hyperglycemia over a 24-week period than the control group.
The FreeStyle Libre system has been approved for use in 30 countries although it is not yet FDA-approved for use in the U.S.; a version of the system is approved for medical professional use. Dunn declined to give any information about a possible timeline for FDA approval of the system for personal use.
Insulin Nation, and its owner, Self Rx, receive no compensation from Abbott or Dexcom for this article. All editorial content is generated independently of any advertising for Insulin Nation and/or Self Rx.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.