(Mainly) Prickless CGM Approved for Kids in Europe
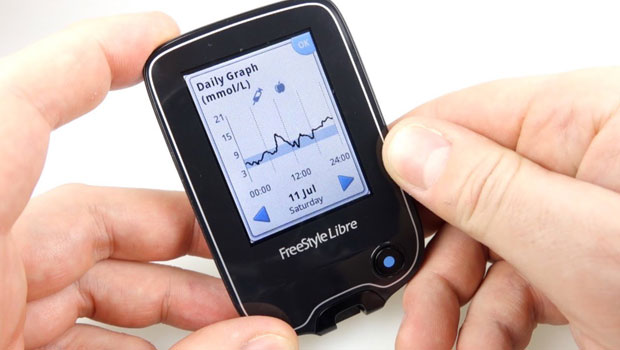
Abbott Laboratories has seen success in Europe with the FreeStyle Libre Flash, a continuous glucose monitoring system which eliminates many blood draws for calibration. The company was originally granted its CE mark to market the device to adults in 2014; the CE mark is like FDA approval in the U.S. Now European regulators have approved the use of the Flash system for children as young as four with Type 1, according to a FierceMedicalDevices report.
In a recent survey of the product, Abbott’s Flash showed positive clinical results in consistency, accuracy, and stability for up to fourteen days without the need for any additional finger prick calibration. That’s a major selling point that the Illinois-based company hopes to use now to gain FDA approval. Abbott released a statement announcing the expanded CE mark, and telegraphing its next move to approach U.S. regulators for approval. In an interesting, if heavy-handed, twist, the statement even quoted a worried U.S. mom of a child with Type 1 advocating for the Libre.
“I still wake up in the night to check my daughter’s blood glucose level with a [finger prick]. I’m very much looking forward to the day that I can use a FreeStyle Libre instead,” the U.S. mother is quoted as saying, using phrasing typical of a press release.
If the Libre’s results hold up in continued testing, such a device would be a welcome addition to the U.S. market. Many medical device manufacturers will introduce innovative diabetes technology in other parts of the world before approaching U.S. regulators, and some elect not to introduce the device in the U.S. at all. CE mark regulations often set a lower bar for approval than FDA regulations, and European regulators are more comfortable with patient risk than U.S. regulators. While FDA approval is considered the gold standard for patient safety, many in the U.S. diabetes community grow frustrated with the pace of device approval.
While Abbott did not reveal a timeline for when it would seek FDA approval, company officials seem to be prepping stockholders and market watchers that the company will approach U.S. regulators soon.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.