Tandem t:slim Pump Approved for Children Ages 6 and Up
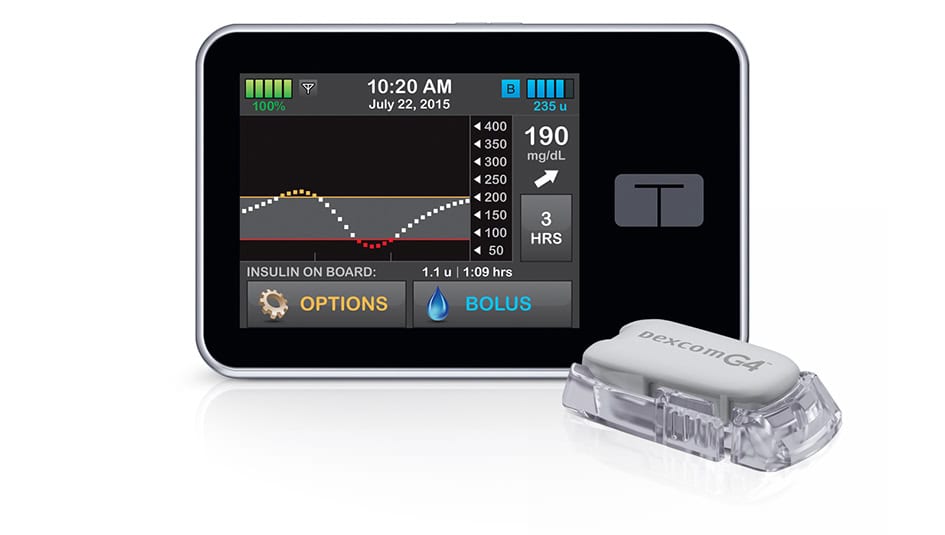
This past month, Tandem announced that their t:slim line of insulin pumps received FDA approval for children as young as 6 years old; previously, children 12 years old and older were approved to use the pump. This approval covers the t:slim, t:flex, and t:slim G4 pumps.
This is good news for parents who are looking at options for pump therapy for little ones. One of the concerns about pump therapy with children is that they lack the surface size to attach a pump and/or a CGM. t:slim pumps are built to be thinner than other pumps on the market, which might make them an appealing choice to try with young children.
This might also be good news for Tandem as it tries to compete with Medtronic, a company reported to hold 74 percent of the market share for insulin pumps in the U.S, according to a 2013 JDRF report. 2016 has been a rough year for Tandem, as the value of its stock has plummeted 40 percent since the start of the year. The biggest dive in value occurred after UnitedHealthcare announced it would make Medtronic the preferred pump company for those it insured.
Tandem officials have long argued that the t:slim’s thin pump design and touchscreen interface offer a superior design to other pumps on the market, and the t:slim has gained a small, devoted following among pump users. Many artificial pancreas researchers have also included the t:slim in clinical trials. It will be interesting to see if this newest FDA approval will expand the market for the t:slim or increase the number of young children with Type 1 on pump therapy.
Insulin Nation did not receive any compensation from Tandem for this story, nor any promise of quid pro quo compensation in the future.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.