FDA OKs AI Device That Detects Diabetic Retinopathy
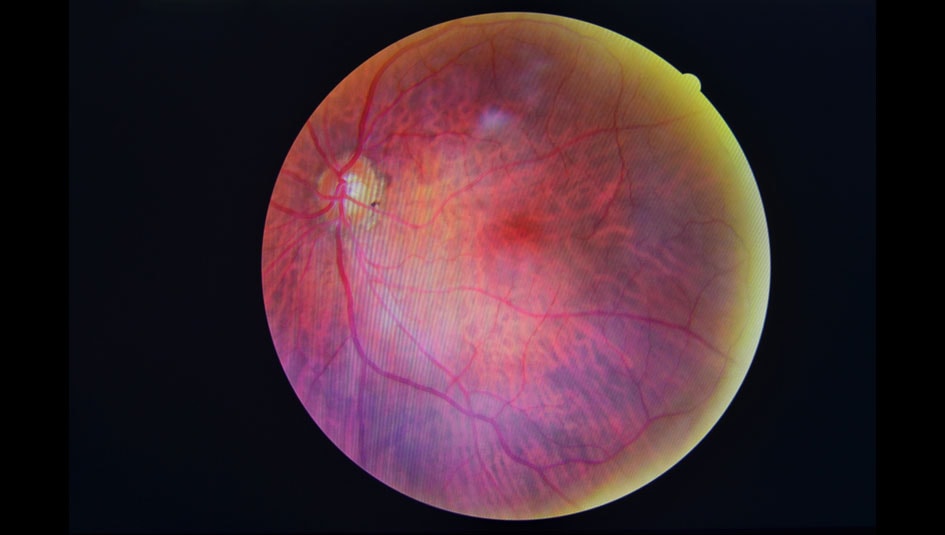
The Food and Drug Administration (FDA) just announced approval of the first artificial intelligence medical device that can detect above mild levels of diabetic retinopathy in adults with diabetes. The device, IDx-DR, can be used by non–eye care professionals, such as primary care physicians. It was in the FDA’s de novo premarket review pathway, which expedites certain novel, low- to moderate-risk devices.
The device issues a screening decision independent of a clinician. In other words, no expert is needed to interpret the image or results. This technology will ideally benefit the many adult patients with diabetes who do not see an eye doctor regularly.
How does it work? The device analyzes images of the retina taken with a high-tech retinal camera. Then, a physician uploads the images to a cloud server with the IDx-DR software. Provided the images are of sufficient quality, the software will issue one of the following results: “more than mild diabetic retinopathy detected: refer to an eye care professional” or “negative for more than mild diabetic retinopathy; rescreen in 12 months.” If the former, the patient should be referred to an ophthalmologist for further diagnostic evaluation.
Before approving the device, the FDA reviewed data from a 900-patient study of retinal images, in which the IDx-DR correctly identified the presence of more than mild diabetic retinopathy 87.4% of the time. The device correctly identified patients with less than mild diabetic retinopathy at an even higher rate (89.5%).
There are certain restrictions for the device. The FDA specifies that patients who have undergone laser treatment, surgery, or eye injections should not use the device. Patients with the following eye conditions may also be ineligible: vision loss, blurred vision, floaters, macular edema, severe nonproliferative retinopathy, proliferative retinopathy, radiation retinopathy, or retinal vein occlusion. Finally, patients who are pregnant should not be pre-screened with the IDx-DR. This is because diabetic retinopathy can develop at an accelerated rate during pregnancy.
Do you have an idea you would like to write about for Insulin Nation? Send your pitch to submissions@insulinnation.com.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.