Stemming the Tide of Immune Response
The next time you get a paper cut, or nick yourself while chopping vegetables, think of the ensuing reaction as a lesson in how your body’s immune system protects you from harm. We bleed when we’re cut not only because the skin covering the outer layers of tissue has been pierced, but also because the body reacts immediately by signaling the blood vessels in the affected area to dilate. This allows an increased flow of blood to the cut as a first line of defense in combating foreign matter. Increased blood flow causes inflammation, which is part of our body’s immune response, protecting the site from biological invaders and promoting healing of the cut.
Inflammation, not the cut, prompts the immune system to act.
Dr. Norma Kenyon, who specializes in transplant immunology, has spent a good part of her career studying inflammation and its effects on Type 1 diabetes. She is the Martin Kleiman Professor of Surgery, Microbiology, Immunology, and Biomedical Engineering at the University of Miami’s Miller School of Medicine, and a Senior Scientist at Miami’s Diabetes Research Institute. In 2012, she was awarded a grant of almost $10 million by the National Institute of Health to support translational research expanding on her earlier discovery that delivering mesenchymal stem cells (MSCs) during islet transplantation more than doubled islet function, enhanced islet survival and reversed rejection episodes. Embedded in the DRI’s recently announced BioHub “mini organ,” MSCs could lead to long-lasting or even permanent cures for people with Type 1 diabetes. One of the people her groundbreaking research could eventually help is her 20-year-old daughter Laura, a Type 1 who almost died from severe hyperglycemia at the age of 14 months. Like so many dedicated to finding cures for diabetes, Kenyon is motivated not just by the thrill of discovery, but also by a deep personal need.
Inflammatory Behavior
“To get a full immune response, you first have to have an inflammatory response,” Kenyon explained. “It’s an initial response to some kind of stimulus in the immune system. In the setting of transplantation, it’s the tissue that has been transplanted from another individual. There are molecules on the surface of that tissue that cause your immune system to attack and destroy it.”
“Once the immune system is activated,” Kenyon continued. “it begins to produce soluble factors, which can affect islet function and insulin production , and also be detrimental to their survival.”
“Soluble factors” is a scientific term that explains how the actions of one group of cells can stimulate the creation or call to action of other cells that act on organs in the body in many different ways. The real function of the immune system, Kenyon explained, is to produce an army of T-cells at the site of inflammation, in this case the islet transplant site. Unsuppressed T-cells target cells they see as enemies, in this case newly transplanted beta cells (within the islet clusters) by absorbing and killing them. The process can destroy up to half of the transplanted islets in the first week after their implantation.
Embedded in the DRI’s recently announced BioHub “mini organ,” MSCs could lead to long-lasting or even permanent cures for people with Type 1 diabetes.
“It’s almost like a foreign body reaction,” said Kenyon. “You get something in your finger: it turns red; it turns warm. That’s an initial inflammatory response, but it’s not specific. The immune system isn’t saying, ‘That’s a splinter,’ or ‘That’s an insulin-producing cell.’ It’s just saying that there is something there that doesn’t belong there. Defensive cells are turned on, and those cells activate and grow and divide. So inflammation is very important, and we want to target that reaction to allow more of the insulin-producing cells to survive in the very beginning. We also want to decrease the potential for a full immune system attack that could lead to rejection and the recurrence of diabetes.”
Fighting Factors with Factors
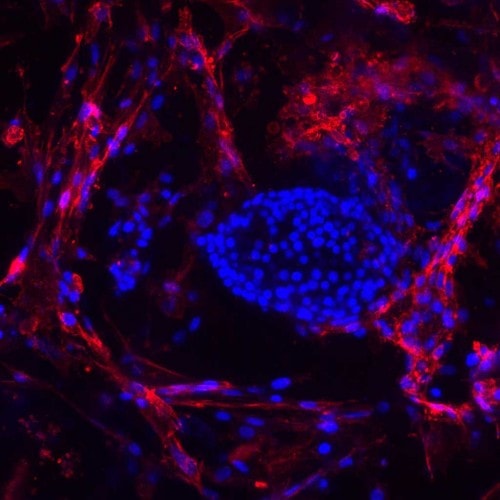
Since inflammation causes immune system response that destroys precious transplanted islets, anything that can stop or slow down the process of destruction is welcome. Kenyon and her research team look at novel and existing drugs that can limit an inflammatory response by targeting either the cells or the soluble factors that are involved in inflammation. Her work with MSCs involves fighting one set of soluble factors with another.
MSCs produce soluble factors that suppress inflammation and immune response, as well as stimulate blood vessel formation and tissue repair. “MSCs have been shown to have a very potent anti-inflammatory effect,” she said. “They have also been shown to help stimulate tissue regeneration. We are co-transplanting those MSCs with islets in order to try to enhance the engraftment of the insulin-producing tissue. We also want to limit the inflammation that occurs so that we can potentially, once we figure out the best approach, use less immune suppression and allow the islets to survive longer.”
MSCs and the BioHub
While MSCs infused into the body along with islets have proven effective to a point, a better therapy may be finding ways to contain and protect both sets of cells once they’re implanted. The DRI’s recently introduced BioHub—a sponge-like silicone wafer packed with islets and designed, after transplantation, to act as a mini-organ, replacing the non-functioning pancreas in Type 1 diabetics—provides a protective scaffolding that keeps insulin-producing beta cells and MSC helper cells together.
“You need to have some kind of housing for those insulin-producing cells,” said Kenyon. “The house is going to have to be built of materials that don’t stimulate inflammation, and has to allow insulin to come out and keep other harmful things from coming in. If you build a really good house that doesn’t stimulate inflammation, it might also have sprinklers that deliver immunomodulatory agents locally so people don’t have to take anti-rejection drugs by mouth and get their systemic side effects.”
There’s No “I” in “Team”
Kenyon doesn’t work alone, and she extols the virtues of team science. The research performed by Dr. Cherie Stabler, a DRI biomedical engineer (see Small Wonders on a Cellular Battlefield) is vital to the success of her own work. “I don’t have the background to design the (BioHub) scaffold, and Dr. Stabler doesn’t have the translational background in the larger animals and the clinical applications that I have,” Kenyon said. “Together, however, we’ve been able to show the best data that I’m aware of internationally for long-term survival of insulin-producing cells in a scaffold.”
Another DRI scientist, Dr. Alice Tomei (see Games Cells Play: Protecting Islet Transplants), has developed conformal coating techniques to “shrink wrap” islets before inserting them into either the BioHub or the body. The BioHub project can incorporate the powerful immunoregulatory and tissue repair properties of MSCs with conformally coated islets, to enhance protection from rejection and recurrent autoimmunity.
Still another member of Kenyon’s team has developed a way to label the MSCs with magnetic markers – nanoparticles that have a magnetic signal trackable using MRI technology – to track their movements and actions once inside the transplant recipient. It isn’t enough just to know that MSCs help prevent inflammation; the researchers also have to know why. “If you have an approach that you’ve found to be effective, but you don’t really understand how it works, you want to track where the cells go when you put them in the body,” said Kenyon. “That can help you understand how they mediate their positive effect.” Initial experiments in test tubes showed that the process achieved good labeling and did not affect the viability of stem cells or their ability to grow and divide. Further transplantation testing with mice has been similarly successful. Tracking markers in larger models like pigs, though, will require more powerful magnets.
Of Mice and Men
So far, both Kenyon’s MSC therapy and Stabler’s scaffolding have shown very promising results in large preclinical models. Tomei’s conformal coatings have been tested and have worked in mice. Her next step will be to test the coatings in larger models.
“We have shown that you can put one of these scaffolds into a clinically relevant model and have function of the insulin-producing cells for more than a year”.
Both mice and pigs, among other preclinical models, have insulin-producing and immune system responses that are quite similar to those in humans. “These experiments are very important for defining direction,” Kenyon said. “Testing in large preclinical models, prior to moving to humans, allows us to see how recipients with immune systems that more closely approximate human’s will react. This data is often essential in order to obtain FDA approval for human trials.”
Kenyon is optimistic about the progress of her work and its ability to cure Type 1 diabetes. “We have shown that you can put one of these scaffolds into a clinically relevant model and have function of the insulin-producing cells for more than a year,” she said. “We’ve cured diabetes in mice more than 250 times. Can you transplant islets using the scaffolds into larger, clinically relevant models and show long-term function and survival of the insulin-producing cells? The answer to that is ‘Yes’.”
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.