CGMs Can Be Durable Medical Devices, CMS Rules
On January 12, the Centers for Medicaid and Medicare laid out criteria for a continuous glucose monitor (CGM) to be considered a “durable medical device.” In doing so, federal regulators opened up the possibility that Medicaid and Medicare programs could cover at least some of the cost for qualifying CGMs and supplies.
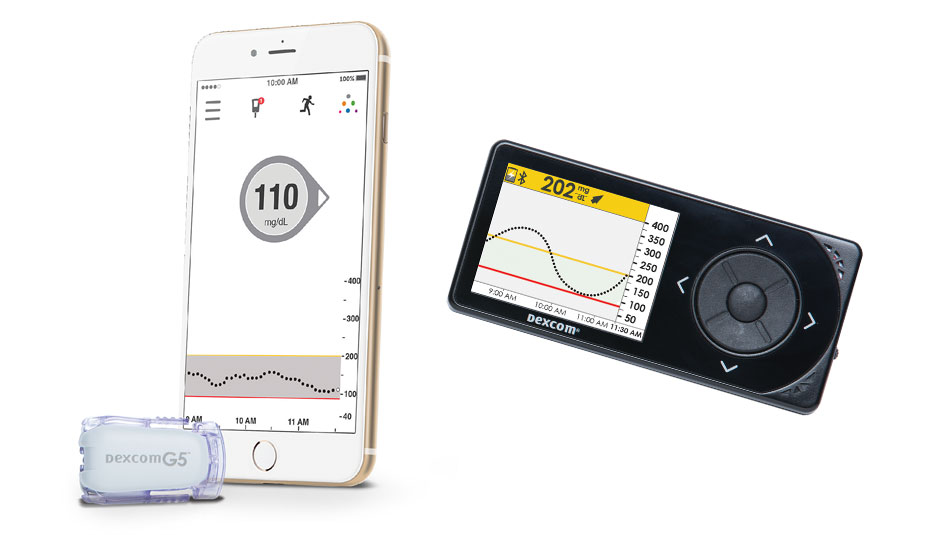
The ruling makes clear, however, that not all CGMs qualify for this new distinction. Only those that the FDA declared accurate enough to be a replacement for fingersticks in making decisions on insulin dosing will be covered. While most CGM owners already use the devices to replace fingersticks, only the Dexcom G5 Mobile has been declared a fingerstick replacement by the FDA. Therefore, the G5 Mobile currently is the only CGM on the market to be defined as a durable medical device under the new CMS ruling.
Read “FDA Approves First Receiver-less CGM.”
This rule change comes less than a month after the FDA decision on the the G5 Mobile, and one can assume that the FDA ruling influenced the timing of the CMS decision. This past year also saw FDA approval of the Medtronic 670G, the first automated insulin pump system on the market; the 670G technology depends upon an integrated CGM for making automatic insulin delivery adjustments. Such regulatory wins for CGM technology most likely weakened the case for denying coverage for CGMs.
The CMS ruling makes clear that CGMs not approved as replacements for fingersticks still will be considered “non-therapeutic” CGMs. According to the FDA, these CGMs should officially only be used to supplement fingerstick readings. It seems likely, however, that CGM makers will now set the bar for the same FDA approval granted to the G5 Mobile, opening up the possibility for more CGM models to be covered by CMS in the future.
It appears that CMS will not cover the full cost of purchasing a CGM, however. Under the new guidelines, CMS will pay between $236 and $277 for a CGM receiver covered under the FDA criteria in 2017. A Google search on January 12 finds that the Dexcom G5 Mobile transmitter alone is priced above $700. CMS also will cover some costs for the replacement of sensors, transmitters, and “all other accessories and supplies essential for the effective use of the receiver.” The monthly coverage for supplies for a qualifying CGM will be $248.38 in 2017. The press release states that coverage limits for both the CGM and supplies will rise in 2018.
Read “FDA Panel Recommends Expanded CGM Use.”
It should be noted that this ruling comes in a time of uncertainty for Medicare, Medicaid, and the health insurance industry. Republican lawmakers who will control Congress and the Executive Branch have vowed to repeal and replace the Affordable Care Act, which expanded Medicaid coverage in many states, in 2017. It is currently impossible to know how changes lawmakers make to health care policy might affect CMS coverage.
You can read the CMS ruling here.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.