The Hygiene Hypothesis: Too Clean for Our Own Good?
At 16, Karin Hehenberger was a rising star on the international tennis circuit. Playing for the Swedish National team, she was competing, in her own words, “almost professionally.” Then Type 1 diabetes came calling, derailing her tennis career and changing the course of her life. Today, armed with both Ph.D. and M.D. degrees, a new kidney from her father and a transplanted pancreas, she is diabetes-free. Dr. Hehenberger is also determined to help others either cope with or avoid what she could not- the unexplained onset and destructive power of autoimmune diseases.
Dr. Hehenberger is Chief Medical Officer of a Massachusetts-based company called Coronado Biosciences (www.coronadobiosciences.com). Coronado’s senior management team looks like it should be attached to a much bigger company. Its recently appointed CEO, Dr. Harlan Weisman, was Chief Science and Technology Officer for Medical Devices and Diagnostics at drug behemoth Johnson and Johnson until late in 2012. The company’s science, along with the therapeutic products it has in development, are built around a logical extension of a relatively recent theory known as the Hygiene Hypothesis.
Originally put forward by Dr. David Strachan in the British Medical Journal in 1989, the hygiene hypothesis observed that hay fever and eczema, both autoimmune conditions, were less common in children from large families. Presumably, this happened—or didn’t happen—because children from large families had been exposed to more infectious agents through their siblings, unlike those without siblings. Transported from its allergic roots, the hygiene hypothesis has since been used by Dr. Joel Weinstock, of Tufts University, to explain a rising tide of autoimmune conditions ranging from Crohn’s disease to multiple sclerosis to Type 1 diabetes, in both developed and rapidly developing countries. Not surprisingly, Dr. Weinstock is a member of Coronado’s Scientific Advisory Board.
Worms to the Rescue

Here’s the theory in a nutshell: Obsessed with cleanliness and hygiene, developed parts of the world have done such a good job of purifying water; pasteurizing milk; inoculating against smallpox, measles, mumps and other infectious diseases; and generally promoting germ-free environments, that our immune systems are left with little to do, and forget or stop doing what centuries of evolution had taught them. So they turn on us, destroying healthy cells and promoting autoimmune conditions like diabetes and multiple sclerosis. In addition, lack of exposure to parasites and resulting vulnerability to autoimmune diseases may be passed on to our children while they are in the womb. This might explain why diagnoses of Type 1 diabetes, for example, have increased significantly in very young children over the past 30 years.
The solution: re-expose our bodies to parasitic pathogens in measured doses, to “reset” our immune systems, and get them to do what they should be doing, which is killing foreign parasites. The principle is similar to that of inoculation, whose preventive effects are well known.
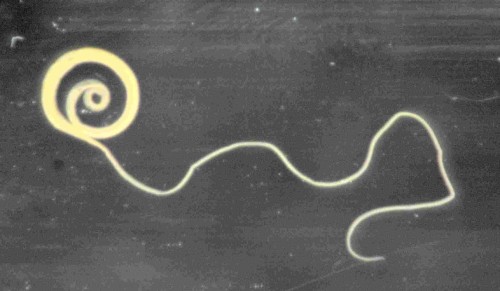
Coronado’s efforts focus on using TSO, an acronym for Trichuris suis ova, or the eggs of a pig whipworm, to retrain the body’s defense mechanisms. TSO is tiny, observable using only high magnification. Retrieved from pig stools and sanitized, they can be distilled into a saline solution that trial subjects drink. The liquid is both odorless and tasteless.
TSO is a promising therapy because they never enter the bloodstream, cannot multiply in human subjects, and are digested and discarded after a few weeks.
The worms appear to have three major impacts on the immune system. They cause changes that activate regulatory T-cells, which reduces immune response, and they seem to prevent activation of “effector” T-cells that, left unchecked, will destroy healthy cells they see as foreign. They also seem to reestablish the normal microbiome in the stomach which help maintain intestinal health. With the permission of both the FDA and European Medicines Agency, Coronado is studying TSO as a potential treatment for various autoimmune conditions.
So far, early trials have shown positive responses in patients with Crohn’s disease, ulcerative colitis and multiple sclerosis. The next planned round of trials will continue to test TSO therapy on these diseases, as well as psoriasis and autism, while trials in subjects either recently diagnosed with Type 1 diabetes or at risk of developing Type 1 should begin later this year in two U.S. locations and one overseas.
Prolonging the “Honeymoon Period”
People at risk of developing Type 1 diabetes can be reliably identified by blood tests showing the presence of specific auto-antibodies. Screening is primarily focused on children and adolescents, particularly those whose parents or siblings have the disease, and is being done right now in programs under the guidance of TrialNet (www.diabetestrialnet.org). Presence of only one auto-antibody does not guarantee that a child will eventually become a Type 1, but there are mathematical formulas for assessing an individuals risk.for becoming Type 1 since there are more auto-antibodies in the serum as blood sugar starts to increase. The way Coronado and other companies are planning to measure the effect of any therapy, including TSO, is to monitor the subjects over a long period, while also working to identify so-called surrogate markers, which may help to speed up the timeline for regulatory approval..

The effects of TSO therapy in recently diagnosed Type 1s will be somewhat more straight forward to measure. Many people with Type 1 diabetes go through a “honeymoon period,” in which beta cells that have survived the first onslaught of the immune system continue to produce insulin. Introducing a therapy like a bi-weekly drink of TSO-laden saline solution might prolong the honeymoon, and create a more stable metabolic situation. The hoped-for result would be reducing the amount of external insulin needed, potentially delaying full dependence on injections for years, and perhaps preventing some of the late-stage complications often associated with long-standing diabetes. In a very optimistic scenario, the TSO solution might even preserve some beta cells long enough for an artificial pancreas or a biological cure to become a reality.
Assuming it is shown to be effective over the next several years of trials, the only drawback to TSO therapy is that no one knows what might happen if or when patients stop their bi-weekly doses. In that sense, a cynic might observe that all we are doing is substituting one therapy for another. True enough, but for Type 1 patients, even the sharpest needles and the most minimal discomfort from injected insulin are very far away from living with a normal pancreas. Karin Hehenberger has seen both sides, and we know which one she prefers.