A Simpler Approach To Treating Hypos
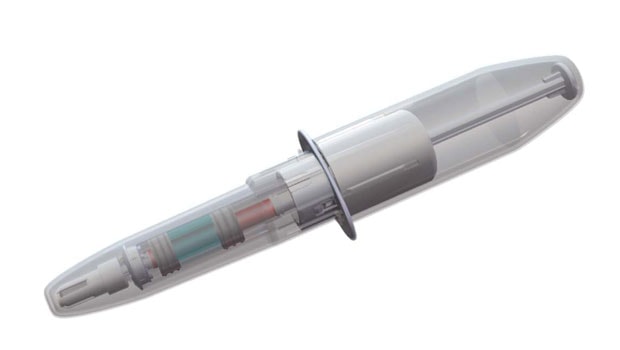
A story about the development and testing of a simpler, more intuitive glucagon-based hypoglycemia rescue device at first seems straightforward. Connecticut-based Biodel (www.biodel.com) is designing a product that enables users to quickly inject glucagon during a hypoglycemic event using a syringe already loaded with both powdered glucagon and an aqueous solution that mixes the two just by removing the syringe’s protective cover. That may sound somewhat complicated on paper, but it’s meant to be quicker and easier than glucagon rescue kit products on the market.
Scheduled for an FDA New Drug Application submission in 2015, Biodel is another potential entry into an array of glucagon-based products (see “Harnessing Hypos: Glucagon As An Everyday Tool”) that aim to help Type 1s and Type 2s recover from hypos or avoid them altogether.
But digging deeper into the issues surrounding hypoglycemia and how to treat it reveals a complex set of problems that go far beyond enhanced glucagon delivery systems. For most of the modern treatment history of diabetes, hypoglycemia has been like sex in the Victorian era as something understood to happen all the time, but not to be talked about in public. Glucagon, for its part, has been called “the Rodney Dangerfield of hormones”, due to its lack of respect. The same might be said of hypoglycemia, the problem it addresses.
A Pressing Need, Largely Unmet

The value of a glucagon-based product is easy to see. Almost all Type 1s and Type 2s on insulin are at high risk for at least one severe hypoglycemia event (called a SHE) a year. Even many Type 2s who don’t use insulin are at risk for SHEs. The longer one has had diabetes, and the more hypos one has suffered from, the more likely SHEs are in the future.
The problem is particularly pressing among older people with diabetes. Manual dexterity and vision may decrease as one ages, making a complex preparation process for current glucagon rescue kits harder. Since the brain runs on glucose, prolonged deprivation of it from hypoglycemia may increase the chances of dementia and related brain diseases, such as Alzheimer’s or Parkinson’s.
This sounds like a problem that everyone with diabetes and their support teams ought to know about and be ready to pounce on at the first sign of symptoms, but it isn’t. According to multiple sources, less than 10% of all Type 1s and fewer than 5% of Type 2s have a glucagon rescue kit in their homes. Those who do have them often keep them in the refrigerator, not exactly a location that would be obvious for someone trying to help. Worse yet, many existing kits in the home have expired.
All this information points to a multimillion-household market for a better glucagon emergency kit. Now that’s a statistic that should whet an investor’s appetite, but there have been complex market forces at work that slow the momentum of any full-throated marketing push for such kits.
The Urge To Just Call 911
Both Lilly, which introduced the first synthetic glucagon in 1999, and Novo Nordisk have glucagon emergency rescue kits waiting for users in pharmacies across the country. But those who take most existing kits on the market haven’t always gotten the most user-friendly product to use. Karmel Allison, Science Editor for Diabetes Magazine’s “A Sweet Life” website once compared the size of the syringes and needles in both Lilly’s and Novo’s products to the sort of tool Lon Chaney might have brandished at a victim in a vintage horror film.
The packaging of Lilly’s rescue kit has improved considerably since her comments were published, but the size of the syringe and length of the needles is the least of the issues. As Allison also wrote, the glucagon powder has to be mixed with the liquid solution in the kit, then drawn into the syringe, and finally administered to the helpless hypoglycemic victim. Since all of this has to happen in an extremely stressful situation, it often doesn’t happen at all. Instead, the would-be doctor for a day pulls out his or her cell phone and calls 911. The costs add up quickly.

To be fair, both Lilly and Novo know how to design appealing solutions that are user friendly. In fact, Lilly recently introduced an iPhone and Android app called “Glucagon”, a well-designed free tutorial that explains how to use its rescue kit. But each company’s primary products for diabetes are insulin-based, and it’s difficult for either company to aggressively market a hypoglycemia rescue solution while their principal, most profitable, products are capable of producing the problem in the first place.
Even though Biodel is also developing two ultra-fast insulin products, the company sees no dilemma in raising awareness of hypoglycemia causes and effects, along with providing a solution to the problem. Gerard Michel, Biodel’s chief financial officer and director of corporate development, joined the company in 2007, and has enjoyed a ringside seat for Biodel’s development of all three products, of which the proposed glucagon kit will most likely be first to market.
“At first, we tried to create a liquid [glucagon] formulation that was stable for long periods when refrigerated and could last for a few days at room temperature,” Michel says. “But we found that doctors and diabetes educators really didn’t care much about whether the glucagon was liquid or powdered. What they all wanted was an emergency device that was easy to use, portable, and reasonably small.”

Biodel’s response, still in prototype stage, is a plastic syringe slightly larger than an ordinary injection device that is separated into two chambers. One holds glucagon powder, the other an aqueous solution. The act of removing the protective cover triggers reconstitution. The theory behind that design is that it is intuitive, even in an emergency situation, to take the device out of its protective cover. Once that is done, all a user needs to do is remove the needle shield and inject. The device is small enough to fit discreetly in a man’s pocket or a small purse, and will have a shelf life of more than a year before it needs to be replaced.
Michel believes FDA clearance will not be a long, drawn-out process, since Biodel’s offering will be similar in many respects to the existing Lilly and Novo products, and the FDA counts heavily on similarity of effect when reviewing new drugs and delivery devices. Biodel anticipates conducting a single dose crossover trial to demonstrate that its glucagon product has similar pharmacokinetic and pharmacodynamic profiles as one or both of the current products on the market. (Pharmacodynamics is often described as the study of what a drug does to the body, whereas pharmacokinetics is the study of what the body does to a drug.)
The Cost Of ER Response
Given the difficulties experienced by non-expert responders to a hypoglycemic event in progress, not to mention a reasonable fear of liability, it’s understandable that a large number of bystanders call 911 and ask for help from trained first responders. While the paramedics may save many lives, the care doesn’t come cheaply.
In recent parallel studies presented by the American Diabetes Association’s Scientific Sessions and the European Association for the Study of Diabetes, researchers revealed the following data about Type 1 and Type 2 hospital admissions for hypoglycemia:
- There were 326,935 admissions listing a primary or secondary cause as Type 1 diabetes in 2009, of which 20,839 were for hypoglycemia. Type 1s admitted for hypos remained in the hospital more than 7 days. At $46,000 per patient, their care cost 33% more than the care given to others hospitalized for diabetes complications not related to hypoglycemia. Medicare paid for approximately 66% of that care.
- Similar statistics for Type 2s in the same year show that 7.2 million people were admitted with Type 2 diabetes as a primary or secondary cause. 228,422 admissions were specifically for hypoglycemic events, with an average length of stay of 7.5 days and a cost of more than $12 billion. Medicare paid for 77% of those claims.
Armed with these statistics, it’s easy to see why in-home treatment for hypoglycemia should be a priority among diabetes caregivers. But this means not only equipping those potentially affected with glucagon rescue kits, but also teaching likely in-place responders (parents and grandparents of youngsters, children of older adults, husbands and wives, co-workers) how to recognize hypoglycemia, use the rescue devices, and give a shot. That’s millions of educational impressions, and it seems a daunting task, bigger than any effort one company could address.
Frontline treatment of hypoglycemia is a huge opportunity to advance care for people with diabetes, but it’s an opportunity that won’t amount to much without an equally huge effort to educate. In a time when health care has increasingly become the concern, responsibility, and domain of the patient rather than the doctor, it is time for a coordinated, multi-party effort to teach people at risk, their loved ones, and their friends, how to save a life.