Will Foot Ulcer Treatment Survive Medicare Rollback?
A state-of-the-art wound therapy that prevents amputations is under threat from proposed cuts in Medicare reimbursement rates, according to regenerative medicine experts.
Organogenesis has pioneered a form of wound care therapy called Apligraf, which uses human skin tissue to create a living dressing to treat diabetic foot ulcers and other stubborn skin wounds. Shire Pharmaceuticals has created Dermagraft, a similar human cell-based dressing that also has become popular for treating diabetic foot ulcers. The two rivals have banded together with other regenerative medicine companies to sound the alarm of an anticipated change in compensation guidelines by the Centers for Medicaid and Medicare Services (CMS).
The new CMS billing structure bundles these more-advanced wound care products with traditional wound care treatments. Currently, a unit of Apligraf costs about $1700, but company representatives warn that it would be bundled under a CMS compensation category that pays roughly half that rate. In an interview with bioflash, Organogenesis CEO Geoff MacKay predicted that the price imbalance would create a disincentive for hospitals to use the treatment. Dr. Lee Rogers, co-director of the Amputation Prevention Center at Valley Presbyterian Hospital, warns that the new compensation scheme would make the newer treatments economically untenable in an editorial for Podiatry Today.
“No hospital or doctor can afford to absorb a $600 loss every time they use a product,” Dr. Rogers writes.
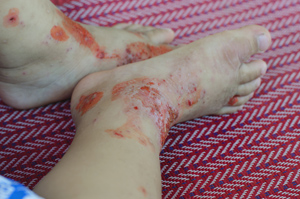
amputations among people with diabetes.
Diabetic foot ulcers are one of the leading causes of amputation for people with diabetes. T1 and T2 populations are at risk for developing foot ulcers because they often experience diminished sensation or loss of sensation in their feet due to neuropathic or vascular damage caused by repeated elevated blood glucose levels. The loss of sensation can cause foot injuries to go unnoticed for those with diabetes who aren’t paying close attention to their feet care. To complicate matters, once injured, the skin of a person with diabetes often has diminished ability to heal.
Studies have shown that this new wave of live-cell dressing works better than other dressings in treating foot ulcers and preventing amputations. A 2012 study conducted by European and Australian researchers at the Diabetic Foot Clinic at King’s College Hospital in London found that Apligraf was nearly twice as effective (51.5% to 26.3%) as traditional wound care in closing diabetic foot ulcer wounds during a 12-week test period, for example. A 2003 study on Dermagraft undertaken throughout 35 U.S. health care centers also showed significant improvement of the treatment over traditional wound treatments during a 12-week period (30% to 18.3%). Both studies also show that diabetic foot ulcers are notoriously difficult to treat, even with the best of treatments, underscoring why it’s important to keep all options for wound treatment on the table, wound care specialists say.
 This uncertainty surrounding the future of wound care billing is also occurring in many other fields of medicine, as CMS is implementing a new payment scheme that moves away from a fee-for-service pay structure into a bundled payment care model in an attempt to incentivize cost-savings while rewarding improved medical outcomes. (It should be noted that the CMS pay structure shift is separate from the changes now being implemented under the Affordable Care Act, also known as Obamacare.) The new price for living-cell wound therapy was part of a 700+page CMS document detailing the new pricing structure; the changed pricings are scheduled to go into effect at the beginning of 2014.
This uncertainty surrounding the future of wound care billing is also occurring in many other fields of medicine, as CMS is implementing a new payment scheme that moves away from a fee-for-service pay structure into a bundled payment care model in an attempt to incentivize cost-savings while rewarding improved medical outcomes. (It should be noted that the CMS pay structure shift is separate from the changes now being implemented under the Affordable Care Act, also known as Obamacare.) The new price for living-cell wound therapy was part of a 700+page CMS document detailing the new pricing structure; the changed pricings are scheduled to go into effect at the beginning of 2014.
In an interview with Insulin Nation, Organogenesis spokeswoman Angelyn Low says the wording of the new pricing rules is still open to interpretation, and regenerative medicine companies are waiting to see how CMS will implement the new pay structure.
However, the impact is already being felt in the industry. In a recent Fierce Biotech article, CEO MacKay warned his staff of “heartbreaking” staff cuts to make the new pricing structure work. Low concedes that major changes will be needed to follow the new CMS standard.
“If it is kept as written, it would certainly mean we would need to change our business model,” Low says.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.