Pump Maker Deals with Quality Control Fallout
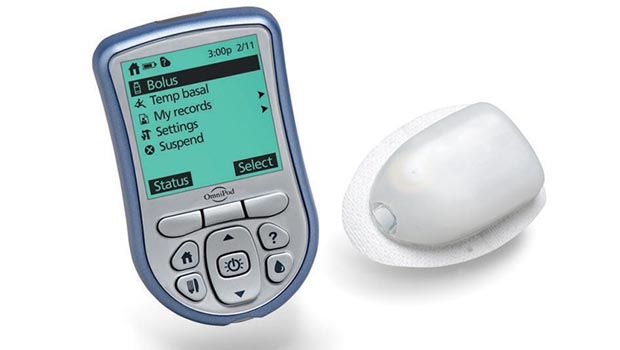
Insulet, known for the OmniPod tubeless insulin pump, recently announced that its manufacturing plant was functioning quite nicely again after a rocky period of quality control issues. This announcement, of course, came just before the FDA released details of a July recall notice that affected over 400,000 OmniPods.
The recall was issued because regulators in March found that the Billerica, Massachusetts-based pump manufacturing facility had “inadequate standards compliance” issues, according to a Fierce Medical Devices article report. There were 90 reported complications with the OmniPod device, 13 of which required medical intervention, the FDA recall notice explained. This included an instance of the device’s cannula retracting or not fully deploying, which means no insulin delivery. The OmniPod recall notice, which Insulet told customers was voluntary, was only for the OmniPod itself and not for the accompanying Personal Diabetes Manager device that wirelessly communicates with the insulin delivery pod.
In response to concerns of quality control, Insulet brought on Michael Spears, giving him the title of vice-president of quality, regulatory and clinical affairs. Spears held a similar position with Covidien, another Massachusetts-located medical device supplier. Insulet has also implemented improved quality control procedures and have received the FDA’s blessing to continue production.
It’s often said that timing is everything in business. It appears Insulet was extremely unlucky in the timing of its public relations moves.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.