Novo Nordisk Recalls More than 70,000 Glucagon Kits
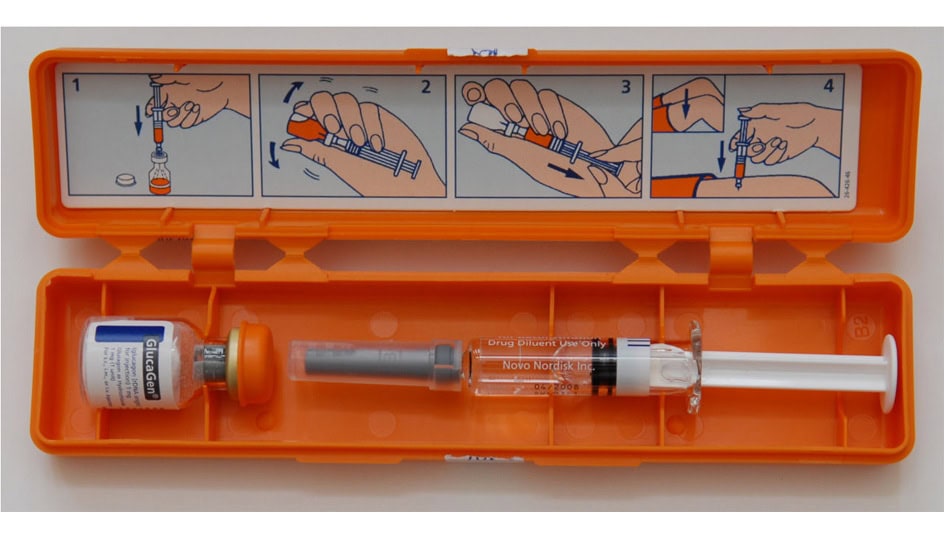
The injectable glucagon kit is considered a weapon of last resort in combatting a bout of hypoglycemia. In fact, many people who care for those with Type 1 choose to call 911 and forgo using the glucagon kits altogether. These kits are not considered user-friendly, as they often require someone who may not be comfortable with needles to mix the glucagon solution and inject it into someone who may be unconscious…oh, and all during a crisis situation.
And now Novo Nordisk has announced that it is recalling some batches of its GlucaGen HypoKit after two users complained that the needles detached during use. Novo Nordisk’s investigators found that a small number of needles do indeed detach, according to a MPR report.
In total, just over 70,000 needles are being recalled from the following four batches:
Batch: FS6X270, Expiry: 09/30/2017
Batch: FS6X296, Expiry: 09/30/2017
Batch: FS6X538, Expiry: 09/30/2017
Batch: FS6X597, Expiry: 09/30/2017
Batch: FS6X797, Expiry: 09/30/2017
Batch: FS6X875, Expiry: 09/30/2017
All six batches were manufactured after February 15, 2016. Lilly also makes a glucagon emergency kit; this recall does not affect that product.
Perhaps this latest recall will hasten the development of easier-to-use glucagon products. Companies have been working on room-temperature-stable glucagon and a glucagon patch. Also, Lilly has acquired the rights to a formulation of glucagon that can be taken nasally.
Thanks for reading this Insulin Nation article. Want more Type 1 news? Subscribe here.
Have Type 2 diabetes or know someone who does? Try Type 2 Nation, our sister publication.