Faster Insulin: A Heated Exchange

The second law of thermodynamics states that an object at a different temperature from its surroundings creates a flow of energy so that the object and the surroundings reach the same temperature. Insuline Medical, a small, publicly owned, Israeli diabetes company, uses this principle to increase the absorption rate of fast-acting bolus insulin by warming the skin and the layers beneath it in the insulin infusion or injection area.
One of Insuline’s two products, InsuPatch, for insulin pumpers, is now under FDA review. The other, InsuPad, for multiple daily injectors, is being tested by Barmer, the largest sick fund (in the U.S., we’d call it a health plan) in Germany, and will seek approval for U.S. distribution once InsuPatch is approved. Insuline filed for FDA approval of the Patch product towards the end of 2012, and hopes for an FDA response to its InsuPatch IDE (Investigational Device Exemption) by mid-2013.
In the most recent clinical trials, both in the U.S. and Europe, Insuline’s heating devices raise the surface temperature of the skin beneath them to 104 degrees Fahrenheit (40 degrees Celsius). The skin and the layers immediately under it are normally at a temperature of 89.6 degrees Fahrenheit, (32 Celsius) while 98.6 degrees is the normal body core temperature. So, the Insuline devices raise the temperature of the areas immediately around and beneath them by approximately 15 percent.
In the studies conducted so far, transferred heat from either patch or pad significantly increases capillary dilation and blood flow in the affected area, resulting in insulin absorption that can be up to 50 percent faster than absorption without the product
Another Entry In The Fast Insulin Sweepstakes
InsuPatch and InsuPad are among several new approaches to making fast insulin work faster. The products aim not only to increase the speed with which bolus insulin reaches its peak inside the body — which makes pre-meal dosing more convenient — but also to reduce the amount of insulin needed for after-meal glucose control.
Smaller insulin doses mean lower costs for both patient and healthcare payer. The most recent InsuPad study, in Germany, reported a 30 percent decrease in pre-meal insulin doses, while also helping to lower the test subjects’ A1c levels. Another important benefit, demonstrated in the same study, is a 45 percent reduction in hypoglycemic events. Absorption speed is very important here, because it helps to prevent hypoglycemia that can occur in current insulin therapies when relatively slow-moving bolus insulin mixes with basal insulin, driving blood glucose content below acceptable levels.
Glycemic control in diabetics is a two-headed monster, and hyperglycemia is also more effectively countered by rapid-onset insulin that comes closer to acting like its naturally produced counterpart. For reasons that are explained in other articles recently published by IN (A Breathtaking Development: Inhalable Insulin, and Faster Insulin: Short and Sweet), subcutaneous injections take longer to break down in the body than natural insulin, due to manmade insulin’s molecular structure. Intradermal injecting, in which existing fast-acting insulin is put into the body using a very short (1.5mm) needle, also significantly improves absorption in clinical trials. But shots using microneedles are still several years away from introduction.
A Faster Path To Market?
“We’re trying to improve the kinetics of fast insulin, without having to create a new drug,” said Ron Nagar, Insuline’s CEO. InsuPatch and InsuPad are both relatively simple products, and thus potentially capable of earning FDA approval and reaching patients in a much shorter time-frame than is likely for some of the other approaches to making fast insulin faster. Each user’s time to peak bolus insulin action will be different, according to his or her own body physiology and other factors, but the general principle of adding heat to increase both diffusion and absorption is well known. It works because by increasing the blood flow because it speeds up the molecules’ diffusion into the bloodstream of whatever is being injected or infused, and therefore seems to be an almost fail-safe addition to therapy.
In the studies conducted so far, transferred heat from either patch or pad significantly increases capillary dilation and blood flow in the affected area, resulting in insulin absorption that can be up to 50% faster than absorption without the product.
InsuPatch is round and InsuPad is rectangular. The pad covers a skin area of approximately two-by-four centimeters, or one by one and one-half inches. The patch is added to an existing insulin pump (Medtronic products are used in the prototypes) and tailored to the pump’s tubing and insertion catheter set. Heat in the patch comes from the bottom of the catheter pad, which is a different size on each pump with which individual patches might be used.
Regardless of the pump model, InsuPatch’s heat is applied only when bolus infusions occur. The most recent Patch tests apply 104 degree heat immediately after a bolus, in alternating bursts of 10 minutes on /10 minutes off, until heat has been applied for a total of 30 minutes. By that time, the bolus insulin is well on its way to peak action, which in the trials occurs about 47 minutes after infusion or injection.
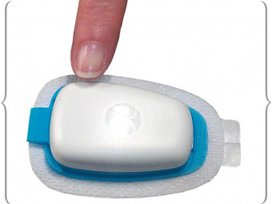
is among several new product approaches
to making fast insulin work faster.
The product is now being tested on
diabetes patients belonging to Barmer,
the largest Sick Fund in Germany.
InsuPad’s rechargeable battery provides enough power to heat the area it covers for four injections, or enough for a normal day’s bolus injecting to cover meals and snacks. When it stops working, it also reminds the user that it’s time to move the injection site, which is a useful benefit that reinforces good site selection and helps avoid tissue damage from overuse of the same area. Likewise, InsuPatch promotes site rotation. Its tubing and catheters are replaced every time the infusion site is moved, usually every three days, a time frame that matches its battery life. Both Pad and Patch will be shipped with a month’s supply of product included.
FDA approval of InsuPatch is the first act of a two-act play. The second part requires Insuline to find a dance partner with an established insulin pump franchise. Part of CEO Nagar’s strategy is to use InsuPatch to add value to a pump in a market that is starting to become crowded after many years of Medtronic dominance. “We’re exploring a number of potential partnership opportunities,” Nagar said..