Medtronic’s “Artificial Pancreas” Gains FDA Approval
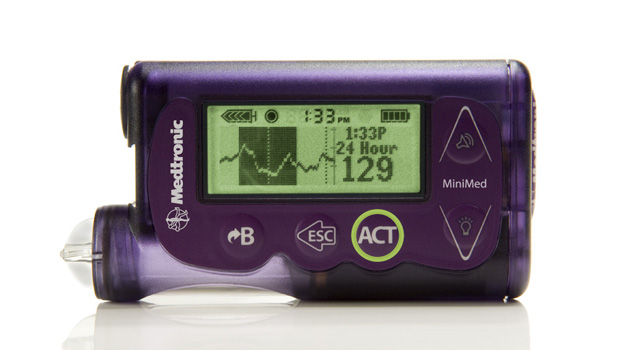
We’ve been trained to believe that when it comes to technology, small companies innovate and big companies commercialize. But rules are made to be broken, and Medtronic’s recent launch of the MiniMed 530g first-generation “artificial pancreas” smart pump shows that this particular large company can innovate, too. The new device and system, which also is designed to stop insulin deliveries automatically when blood sugar levels get too low, just received FDA approval.
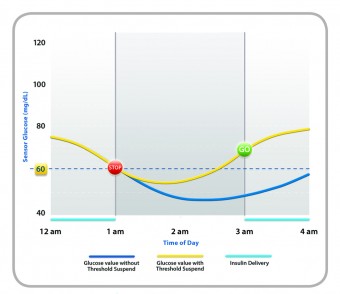
The MiniMed with Enlite, Medtronic’s new sensor technology, combines all of the features of a state-of-the-art insulin pump/CGM combo with a number of new features and one game-changing innovation. When the Enlite sensor detects glucose levels below a limit set by the wearer, it first triggers an alarm, and then, if the wearer doesn’t respond, shuts off basal and bolus insulin for two hours. If the patient is sleeping, doesn’t respond to the alarms, and insulin resumes after two hours, the sensor will once again shut insulin delivery down if another glucose level below the preset number is detected. In effect, the device is doubly fail-safe.
This is the first time the FDA has cleared a device that uses data from a sensor and onboard algorithms to alert patients of the possibility of hypoglycemia, and then suspends insulin doses if the wearer does not react to the warning. It’s also the first example of artificial intelligence being applied to insulin-dependent diabetes therapy that’s been allowed in the U.S. marketplace.
The device is not just important for its own sake, but for what it means to the regulatory environment for artificial pancreases. By clearing the FDA’s stringent approval process under a new category ( “OZO: Artificial Pancreas Device System – Threshold Suspend”) Medtronic has helped to establish a foundation technology against which other AP devices and systems will be measured for U.S. market approval.
Although there are more than 20 artificial pancreas projects underway around the world, and Medtronic’s work has received relatively little fanfare, the MiniMed isn’t a bolt out of the blue. Though Medtronic has been quiet in the unique way publicly-traded companies choose to be quiet about new projects, the Threshold Suspend technology at the heart of the MiniMed device has been available in Europe for almost 3 years (See Insulin Nation’s (“Artificial Pancreas Update: The Leader Checks In”). However, the FDA doesn’t often take patient experiences in other countries into account, so Medtronic needed to make a whole new case to get approval.
While utilizing the Threshold Suspend to stop insulin delivery is a novelty, the MiniMed incorporates several other new tools, as well. Its Enlite sensor is, on average, 31% more accurate than previous Medtronic sensors, detecting glucose levels of 70mg/dl, or below 93% of the time in FDA trials. The device is also significantly smaller than previous Medtronic pump/CGM combos. Finally, Enlite sensor offers a 90-degree insertion needle that makes changing locations simpler and more comfortable.
Right now, MiniMed is approved for patients 16 and older. Medtronic is moving as fast as it can to establish trial protocols to gain MiniMed approval for use by children ages 2 to 16. The company hopes to begin recruiting subjects by the end of 2013. It’s believed that FDA approval for younger patients should go quicker than the initial clearance.
The lack of certainty that surrounded the eventual FDA clearance means that Medtronic waited until it got approval to begin manufacturing MiniMed in large quantities. However, the challenge won’t be ramping up production, but distributing the pumps in a way that allows both medical professionals and patients to learn how to use the new system, says Mike Hill, Senior Marketing Director for the Insulin Delivery Business at Medtronic.
“We don’t want to dump tens of thousands of devices on the market overnight,” says Hill.
The MiniMed is a baby step towards a fully-automated, closed-loop pancreatic system. The device simulates only one aspect of normal pancreatic function, albeit an important one. The prevalence of hypoglycemia among Type 1s makes Threshold Suspend a truly useful and important innovation, and it is a base upon which Medtronic can continue to build devices that offer more automated functions.
Medtronic has not adopted a bi-hormonal (insulin and glucagon) approach for the MiniMed, and this means the company is fully invested in a single hormone approach to pancreatic substitution. But this is good for consumers, as it means that there is a reasonable possibility that a competitive set of products relying on a two-hormone approach will eventually reach the market. Boston University biochemical engineer Ed Damiano and Dr. Steven Russell of the Massachusetts General Hospital Diabetes Center have created a two-hormone bionic pancreas using Dexcom sensors and, eventually, Tandem Diabetes’ two-chamber pump, for example. Competition fosters innovation, and a rivalry between therapies is the best guarantee patients can have that the solutions to their problems will continue to emerge.
But for now, this is Medtronic’s moment, as it is the first out of the gate to get the FDA seal of approval.