Artificial Pancreas: What’s in a Name?
Commentary
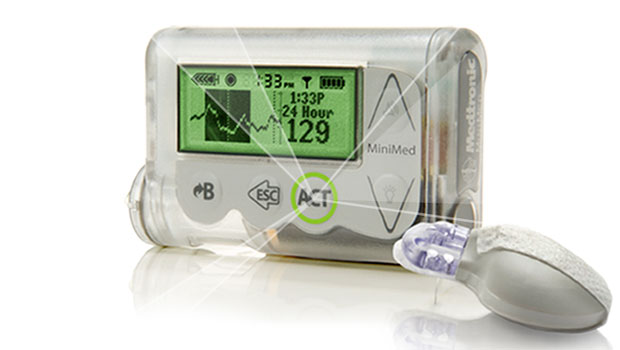
“Artificial pancreas” is a term that can make the T1 community sit up and take notice. It certainly did after a round of recent reports about Medtronic’s new MiniMed 530g pump/CGM. The pump and system, which features a Low Glucose Suspend feature that shuts off basal insulin if blood sugar readings gets too low, has been billed by Medtronic as an artificial pancreas. Most major news outlets (the Wall Street Journal, Reuters, and even Insulin Nation) called the new device and system an artificial pancreas, as well.
But not everyone in the T1 community agreed with that terminology. Within an hour, Insulin Nation columnist “Iron Andy” Holder weighed in via email, saying that he thinks the device doesn’t qualify for the term.
“I don’t know that a pump that shuts off basal insulin when BG goes low should be called an AP. If it administered glucose, that’s a different story,” Holder wrote in an email.
Another regular Insulin Nation reader shared a letter with Holder addressed to Medtronic, chastising the company for calling its new device an “artificial pancreas”:
“Medtronic, I congratulate your effort of the new insulin pump – looks much improved for sure. That being said, I think calling it an ‘artificial pancreas’ system is misleading. This is not an ‘artificial pancreas’ system!…Stating otherwise, simply by use of the term, is misleading to the largely emotionally fatigued and sometimes emotionally fragile members of the D community you serve,” the letter stated.
Holder believes for any device to be called an “artificial pancreas” it should measure blood glucose and administer both insulin and glucagon when needed. Such a device would fundamentally alter the lives of people with diabetes, he said.
But the use of the term is far from settled in public. For example, the Minneapolis Star-Tribune wrote a story on September 27th characterizing the new device as an “artificial pancreas”. The next day it published an article that considered the new MiniMed pump and system as a “step towards an artificial pancreas”.
To be clear, there is no system available now, or in the pipeline, that fully replicates the functions of the pancreas in any person, with or without diabetes. To do so would require a device to govern a complex relationship between beta (insulin), alpha (glucagon), delta (somatostatin), and gamma (pancreatic polypeptide) cells. The interaction between these cells and the nervous system is not even fully understood. If that’s what an artificial pancreas must be, then the MiniMed isn’t it.
Others, like Medtronic representatives, characterize a device like the MiniMed as an artificial pancreas because it automates some pancreatic functions, much like the body does. The biggest value of the MiniMed might indeed be its Low Glucose Suspend, which is a step towards artificial intelligence in regulating insulin in people with diabetes. FDA clearance of the MiniMed 530g is a truly significant first step towards allowing mathematical formulas, rather than human calculations, to control insulin dosing. With this device, there is now a standard against which other, more complex and multi-functional systems can be compared.
Does that make the MiniMed an artificial pancreas? It depends on who you ask, and the debate is reflected on Insulin Nation’s Facebook page. When Insulin Nation posted an article last week characterizing the device as an artificial pancreas, two users chimed in with opposing viewpoints. Australia reader Catherine Forbes wrote, “It’s a long way from an artificial pancreas,” but Minnesota reader Marta B. Hohnstadt said,“I don’t care what you call it, it’s still a step forward!”
As such devices become more common in the marketplace, and as advances in pump technology continue, it’s likely that the terminology, both in the media and in conversations, will settle into consensus in the coming months. (Think of the debate that once occurred between the terms “Internet” and “World Wide Web”.) Until then, the debate continues on message boards, over dinner tables, and even in the editorial meetings of Insulin Nation.
Feel free to join in the debate on Insulin Nation’s Facebook, Google+, LinkedIn, and Twitter pages.